Sodium bisulfate molar mass
Home » chemical » Sodium bisulfate molar mass >Sodium bisulfate molar mass
Sodium Bisulfate Molar Mass. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Modification of work by the Italian voiceFlickr. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Then the resulting homogenate was passed sequentially through 80 and 120 mesh sieves.
Vanadium as V less than 0001. Elements can combine during chemical reactions to form new compounds. Write the names of the two elements in the compound 1 The name of the first element remains the same. Chlorides as sodium chloride 002. Draw the following alcohols heptan-2-ol. It is more crystalline when compared to starch.
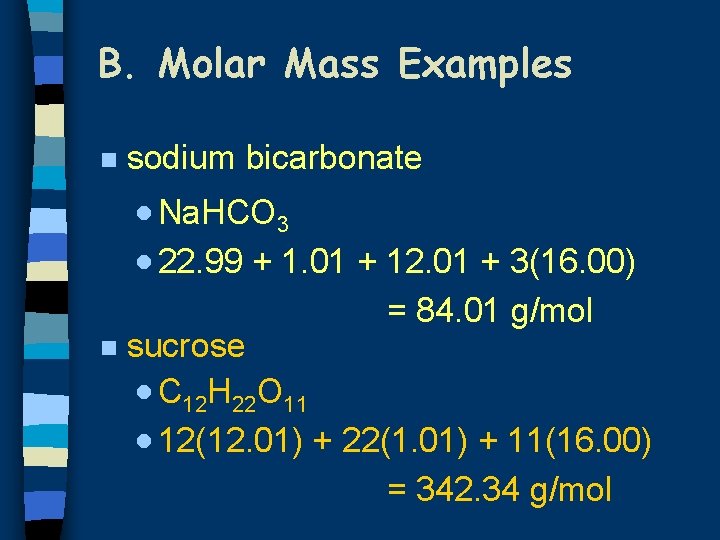
Molar Mass FeOH 3 106.
Potassium carbonate K 2. Such results indicated the concentration of reactants plays an important role in oxidative. Mass spectrum Mass spectrometer Mass spectrometry. Write the names of the two elements in the compound 1 The name of the first element remains the same. Polyethylene glycol molecular weight 200-6000 Polyethylene glycol mono-isotridecyl ether sulfate sodium salt CAS Reg. Sodium sulfate 2Na SO 4 2- Na 2 SO 4.
 Source: slidetodoc.com
Source: slidetodoc.com
What Is An Isomer. 7140 8689 5648 1426 O or. We would like to show you a description here but the site wont allow us. Mass O 38. Among the fuel rich stoichiometric ratio and fuel lean systems the stoichiometric ratio system is most conductive to product synthesis and the mercury removal performance of the obtained product was the best.
 Source: sciencemadness.org
Source: sciencemadness.org
Among the fuel rich stoichiometric ratio and fuel lean systems the stoichiometric ratio system is most conductive to product synthesis and the mercury removal performance of the obtained product was the best. Mass NaF 200 g. Molar mass 201201 291008 1900 31600 33643 gmol Mass C 201201 g C 100 7140 C 33643 g compound Mass H 291008 g H 100 8689 H 33643 g compound 22 CHAPTER 3 Mass F STOICHIOMETRY 1900 g F 100 5648 F 33643 g compound Mass O 10000. H 2 PO 4 Dihydrogen phosphate. Then the resulting homogenate was passed sequentially through 80 and 120 mesh sieves.

This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species. Mass O 38. The results showed that the best synthesized product was when the doping amount of Mn was the molar ratio of 05 and the average mercury removal efficiency was 875 within 120 min. Sodium sulfate 2Na SO 4 2- Na 2 SO 4. Rb 3 N Rubidium nitride d.
 Source: en.wikipedia.org
Source: en.wikipedia.org
0 ____ Formula Mass 164. Molar mass 201201 291008 1900 31600 33643 gmol Mass C 201201 g C 100 7140 C 33643 g compound Mass H 291008 g H 100 8689 H 33643 g compound 22 CHAPTER 3 Mass F STOICHIOMETRY 1900 g F 100 5648 F 33643 g compound Mass O 10000. For example 40 g of rice was soaked in 200 mL sodium bisulfate solution with a concentration of 10 mgmL at room temperature for 12 h and then homogenized using a Joyoung kitchen blender Jinan China at 250 W for 2 min. MnO 4 Permanganate. Among the fuel rich stoichiometric ratio and fuel lean systems the stoichiometric ratio system is most conductive to product synthesis and the mercury removal performance of the obtained product was the best.
 Source: study.com
Source: study.com
Chlorides as sodium chloride 002. Such results indicated the concentration of reactants plays an important role in oxidative. Sulfates as sodium sulfate 041. Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. C 2 H 3 O 2 Acetate.

Structure of Cellulose C 6 H 10 O 5n. Bisulfate Blood sugar Blue shift. Potassium carbonate 2K CO 3 2- K 2 CO 3. Rb 3 N Rubidium nitride d. Surprisingly the catalytic performances on AuHVP catalyst were found to be highly dependent on the molar ratio of MeOH to MAL as the conversion of MAL decreased from 463 to 303 and the selectivity of MMA reduced from 824 to 607 with the molar ratio decreased from 40 to 20 Fig.
Polyethylene glycol molecular weight 200-6000 Polyethylene glycol mono-isotridecyl ether sulfate sodium salt CAS Reg. C 2 H 3 O 2 Acetate. Mass NaF 200 g. How do i calculate the amount of acid to reduce water ph. C 2 O 4 2-Oxalate.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is more crystalline when compared to starch. How do i calculate the amount of acid to reduce water ph. C 2 O 4 2-Oxalate. Then the resulting homogenate was passed sequentially through 80 and 120 mesh sieves. Sodium sulfate Na 2 SO 4.
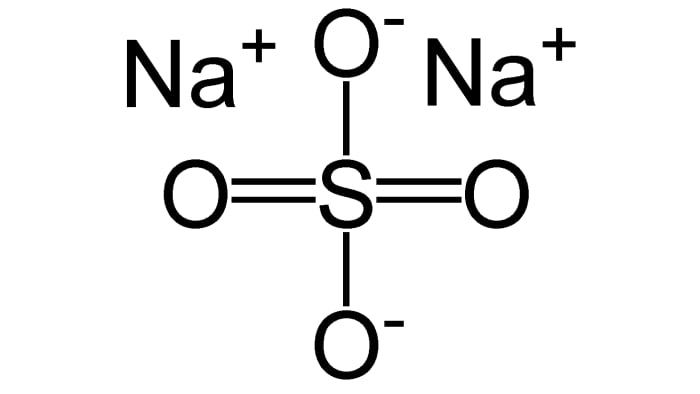 Source: melscience.com
Source: melscience.com
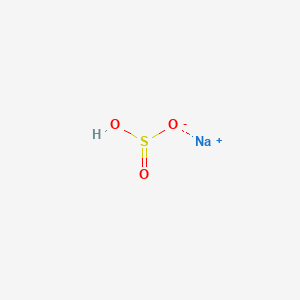
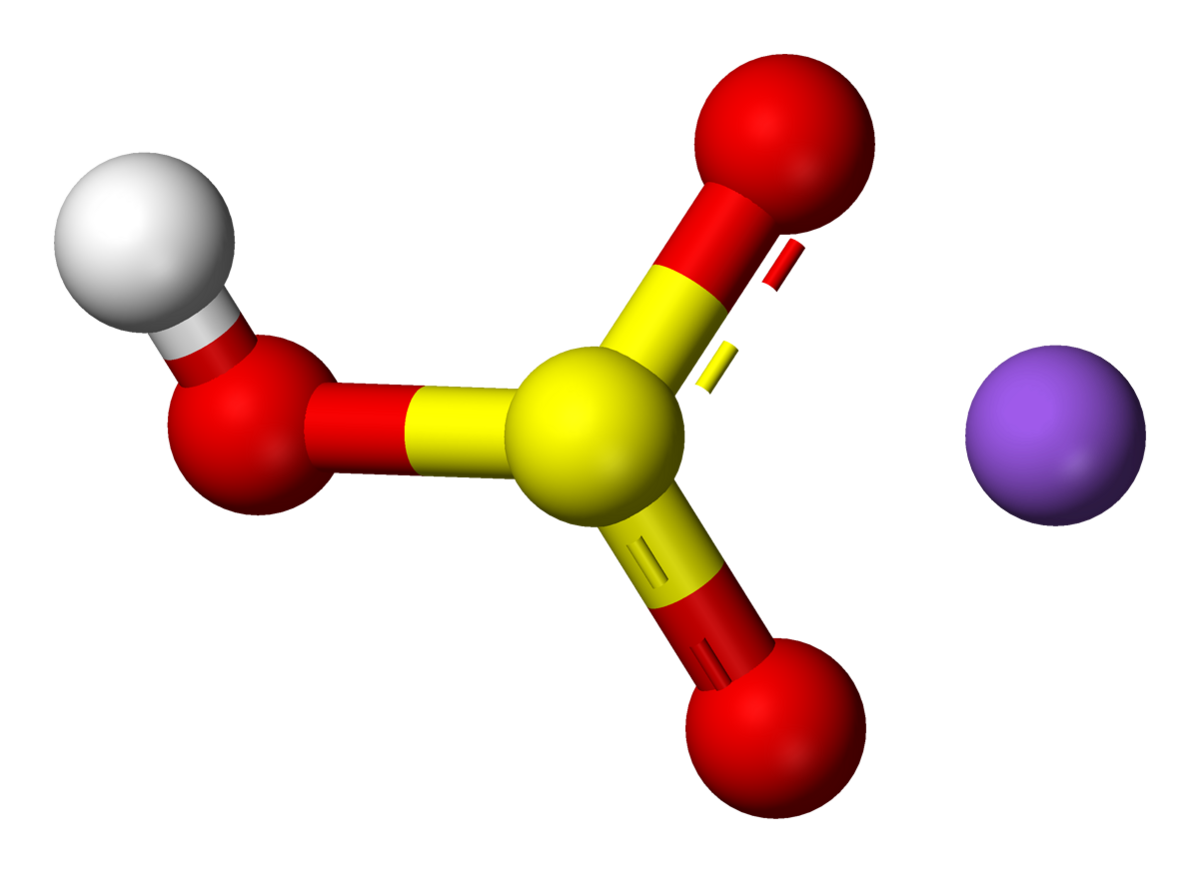
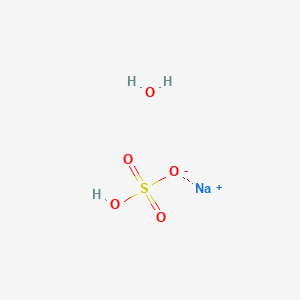
Molar mass 201201 291008 1900 31600 33643 gmol Mass C 201201 g C 100 7140 C 33643 g compound Mass H 291008 g H 100 8689 H 33643 g compound 22 CHAPTER 3 Mass F STOICHIOMETRY 1900 g F 100 5648 F 33643 g compound Mass O 10000. Sodium bisulfate also known as sodium hydrogen sulfate is the sodium salt of the bisulfate anion with the molecular formula NaHSO 4Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium base typically in the form of either sodium hydroxide lye or sodium chloride table salt. Vanadium as V less than 0001. Polyethylene glycol molecular weight 200-6000 Polyethylene glycol mono-isotridecyl ether sulfate sodium salt CAS Reg. Such results indicated the concentration of reactants plays an important role in oxidative.
 Source: melscience.com
Source: melscience.com
Draw the following alcohols heptan-2-ol. Sodium sulfate 2Na SO 4 2- Na 2 SO 4. Sodium sulfate Na 2 SO 4. How do i calculate the amount of acid to reduce water ph. Cr 2 O 7 2-Dichromate.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium bisulfate molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.