Zinc chloride ammonia reaction
Home » chemical » Zinc chloride ammonia reaction >Zinc chloride ammonia reaction
Zinc Chloride Ammonia Reaction. Add a pinch of Eriochrome Black T ground with sodium chloride 100 mg of indicator plus 20 g of analytical grade NaCl. All forms are soluble in water. Zinc nitrate formula. S N 2 examples.
 Zinc Chloride And Ammonium Hydroxide Reaction Youtube From youtube.com
Zinc Chloride And Ammonium Hydroxide Reaction Youtube From youtube.com
For example when zinc reacts with hydrochloric acid hydrogen gas is evolved with formation of zinc chloride. S N 2 Reaction. The first accelerators used were inorganic basic materials. Ammonium chloride is made as a by-product of the classic Solvay process used to manufacture sodium carbonate. Ammonium chloride is then crystallized from the filtrate separated. Stability and structure of carbocations.
The method involves reaction of ammonia carbon dioxide and sodium chloride in water.
The result is a product that is stronger more elastic more resilient and less sensitive to temperature changes and the action of solvents than the original polymer. The music titled Positive Reactions was composed by Lani Elisabeth. The ICSC project is a common undertaking between the World Health Organization WHO and. A new music video featuring our popular chemical reaction footages. NH 4 2 ZnCl 4 and NH 4 3 ClZnCl 4 which decompose on heating liberating HCl just as zinc chloride hydrate does. The following ions form ammonia complexes.
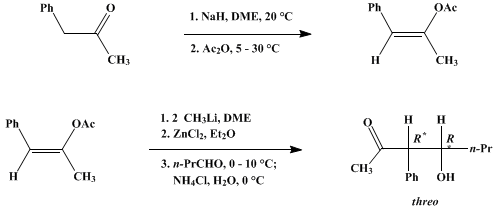 Source: orgsyn.org
Source: orgsyn.org
We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Anhydrous zinc sulfate is a colorless crystalline solid. Change in Color Some chemical reactions are accompanied by a change in color. Add a pinch of Eriochrome Black T ground with sodium chloride 100 mg of indicator plus 20 g of analytical grade NaCl. Sodium bicarbonate precipitates from solution and is recovered by filtration.
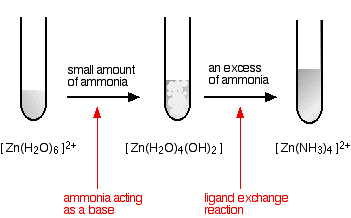 Source: chemguide.co.uk
Source: chemguide.co.uk
Ammonia is a compound of nitrogen and hydrogen with the formula NH 3A stable binary hydride and the simplest pnictogen hydride ammonia is a colourless gas with a distinct pungent smell. Simple S N 2 reaction. The chemicals Indium chloride tetrahydrate InCl 3 4H 2 O thioacetamide C 2 H 5 NS and Zinc dichloride ZnCl 2 were used. The reaction occurred when a piece of metal salt was dropped in water glass. Consult your instructor to be 100 sure that this applies to your course.
 Source: youtube.com
Source: youtube.com
In 2 S 3 and ZnIn 2 S 4 were synthesized using the same. Substrate structure controls substitution mechanism S N 1 or S N 2. The ICSC project is a common undertaking between the World Health Organization WHO and. Ammonium chloride is made as a by-product of the classic Solvay process used to manufacture sodium carbonate. For example when zinc reacts with hydrochloric acid hydrogen gas is evolved with formation of zinc chloride.
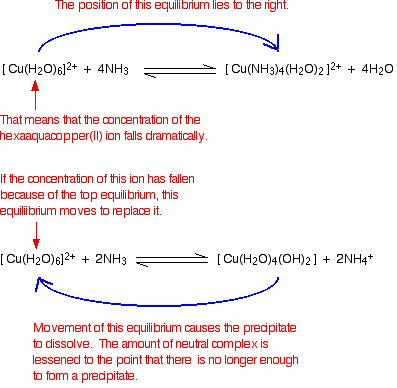 Source: chemguide.co.uk
Source: chemguide.co.uk
Zinc chloride a Lewis acid increases the reactivity of alcohols towards acid. They included basic lead carbonate lime magnesia and. Ammonium chloride is then crystallized from the filtrate separated. Ammonium chloride is made as a by-product of the classic Solvay process used to manufacture sodium carbonate. Immediate steps should be taken to limit its spread to the environment.
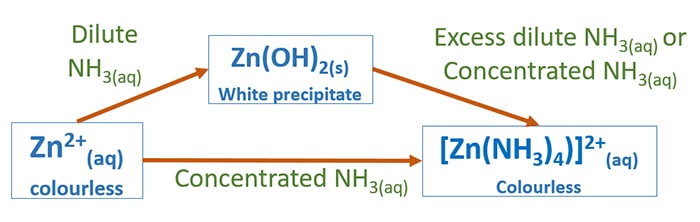 Source: chemistryscl.com
Source: chemistryscl.com
In general catalytic action is a chemical reaction between the catalyst and a reactant. The primary hazard is the threat posed to the environment. They included basic lead carbonate lime magnesia and. The method involves reaction of ammonia carbon dioxide and sodium chloride in water. The reaction occurred when a piece of metal salt was dropped in water glass.
 Source: youtube.com
Source: youtube.com
The chemicals Indium chloride tetrahydrate InCl 3 4H 2 O thioacetamide C 2 H 5 NS and Zinc dichloride ZnCl 2 were used. Reaction - Zn 2HCl ZnCl 2 H 2. Benzyl Chloride with HS S N 2 Reaction. Anhydrous zinc sulfate is a colorless crystalline solid. The use of zinc chloride as a flux sometimes in a mixture with ammonium chloride see also Zinc ammonium chloride involves the production of HCl and its subsequent reaction with surface oxides.
 Source: brainly.in
Source: brainly.in
Add a pinch of Eriochrome Black T ground with sodium chloride 100 mg of indicator plus 20 g of analytical grade NaCl. Beauty of Science. If you take an alcohol and add thionyl chloride it will be converted into an alkyl chloride. S N 2 Reaction. If a metal hydroxide precipitate forms continue adding ammonia.
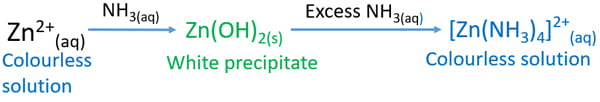 Source: chemistryscl.com
Source: chemistryscl.com
Stability and structure of carbocations. This reagent converts alcohols into the corresponding alkyl chlorides. It is a common nitrogenous waste particularly among aquatic organisms and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world. Dilute to about 100 mL with distilled water. Substrate structure controls substitution mechanism S N 1 or S N 2.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Vulcanization is a chemical reaction between rubber and a functional group usually brought about by heat. Simple S N 2 reaction. All forms are soluble in water. The first accelerators used were inorganic basic materials. Acid Solution Basic Solution Solution with Excess NH 3 Color of.
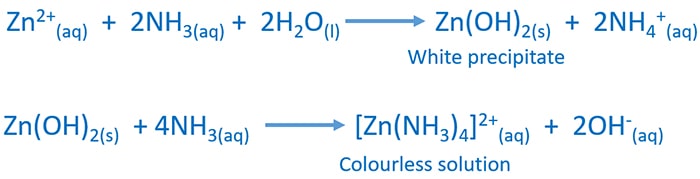 Source: chemistryscl.com
Source: chemistryscl.com
Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. If a metal hydroxide precipitate forms continue adding ammonia. Catalyst in chemistry any substance that increases the rate of a reaction without itself being consumed. Simple S N 2 reaction. The result is a product that is stronger more elastic more resilient and less sensitive to temperature changes and the action of solvents than the original polymer.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title zinc chloride ammonia reaction by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.