When does sodium chlorate become molten
Home » chemical » When does sodium chlorate become molten >When does sodium chlorate become molten
When Does Sodium Chlorate Become Molten. It is generally recommended that PVC is not used for air. For example when each sodium atom in a sample of sodium metal group 1 gives up one electron to form a sodium cation Na and each chlorine atom in a sample of chlorine gas group 17 accepts one electron to form a chloride anion Cl the resulting compound NaCl is composed of sodium ions and chloride ions in the ratio of one Na ion for each Cl ion. Electrolysis of potassium bromide. If PVCU pipe is used in such an instance areas being worked on should be sprayed with a 5050 detergent solution which will.
 And Sodium Chlorate V From studylib.net
And Sodium Chlorate V From studylib.net
The primary metabolic event in the formation of metHb Fe3 from oxyHb Fe2 as a result of. Check the chemical compatibility of LDPE low density polyethylene with various chemicals solvents alcohols and other products. Taken together these findings suggest that it is the presence and cycling of the reductive products of nitrobenzene within RBCs that cause the conversion of oxyhemoglobin oxyHb to metHb. Are shared between from each carbon atom Does not conduct electricity in carbon atoms and is delocalised and free to the. A 010 mole of iron b 231 moles of Si c 00023 mole of carbon d 054 mole of sodium a 56 g. B68 Sodium must be in a molten condition when loaded and allowed to solidify before shipment.
One quarter of pure.
Contact may cause burns to skin eyes and mucous membranes. Impurities like sulfur react with the oxygen to make SO2 gas which then escapes from the molten iron. May ignite upon exposure to air. The conversion ratio of lithium carbonate to lithium metal is. In order to effect decomposition the solid or aqueous solution is added to enough water to make the borohydride concentration less than 3 and then excess equivalents of dilute aqueous acetic acid are added drop wise with stirring under nitrogen. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl.
 Source: chlorates.exrockets.com
Source: chlorates.exrockets.com
Moreover in vitro incubation of RBCs with nitrobenzene does not result in the formation of metHb. Bulk packagings must have exterior heating coils fusion welded to the tank shell which have been properly stress relieved. It is not reactive at room temperatures except by strong oxidizing agents and some solvents cause swelling. Aluminum Chemical Compatibility Chart. More than half of the oxygen that is produced for industrial purposes is used in the process of purifying the molten iron that was made from iron ore.
 Source: en.wikipedia.org
Source: en.wikipedia.org
If the PVC pipe is used for air or blown particles the pipe wall can build up static electricity which can arc to nearby earthed objects and become a fire hazard or health and safety issue for pipe workers. 219 mol Na Krypton really does not give Superman his strength. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. Academiaedu is a platform for academics to share research papers. When fused and electrolyzed at about 840 degrees Farhenheit chlorine gas is liberated while molten lithium rises to the surface collecting in cast-iron enclosures.
 Source: docbrown.info
Source: docbrown.info
Which expression is needed to find the pressure of the O2 if 612 g of the potassium chlorate molar mass 1224 gol decomposes in a rigid 25-L container at a temperature of 525C. LDPE Chemical Compatibility Chart. If you have 000789g of the gaseous element how many moles does this represent. More than half of the oxygen that is produced for industrial purposes is used in the process of purifying the molten iron that was made from iron ore. Na 2CO 3 s 10H 2Ol ¾ Na 2CO 3 10H 2O s Washing soda i Caustic Soda v Plaster of Paris Chemical Name.
 Source: sciencedirect.com
Source: sciencedirect.com
If 504g of sodium is used how many moles of sodium does that represent. Ammonium chloride reacts with sodium hydroxide b. Sodium borohydride NaBH 4 is so stable in water that a 12 aqueous solution stabilized with sodium hydroxide is sold commercially. Aluminum Chemical Compatibility Chart. More than half of the oxygen that is produced for industrial purposes is used in the process of purifying the molten iron that was made from iron ore.
 Source: chlorates.exrockets.com
Source: chlorates.exrockets.com
In order to effect decomposition the solid or aqueous solution is added to enough water to make the borohydride concentration less than 3 and then excess equivalents of dilute aqueous acetic acid are added drop wise with stirring under nitrogen. The chemical formula of ammonium sulfate ie - the formula unit is NH 4 2 SO 4. Heat 2 NaHCO 3 ¾ Na 2CO 3 H 2O CO 2 Common salt is an important raw material for various Sodium hydrogen Sodium Water Carbon carbonate carbonate dioxide materials of daily use like sodium hydroxide baking soda washing soda bleaching powder etc. The pure lithium produced is wrapped in paraffin wax to prevent oxidization. One quarter of pure.
Source: aip.scitation.org
It is generally recommended that PVC is not used for air. It is generally recommended that PVC is not used for air. One quarter of pure. 219 mol Na Krypton really does not give Superman his strength. Water purification also relies on chlorine to kill bacteria in the water after the impure water has passed through various filtration stages.
 Source: en.wikipedia.org
Source: en.wikipedia.org
219 mol Na Krypton really does not give Superman his strength. Modification of work by Mark Blaser and Matt Evans Watch this video to see a mixture of salts melt and. Contact may cause burns to skin eyes and mucous membranes. If you have 000789g of the gaseous element how many moles does this represent. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl.
 Source: studylib.net
Source: studylib.net
The pure lithium produced is wrapped in paraffin wax to prevent oxidization. When fused and electrolyzed at about 840 degrees Farhenheit chlorine gas is liberated while molten lithium rises to the surface collecting in cast-iron enclosures. Sodium borohydride NaBH 4 is so stable in water that a 12 aqueous solution stabilized with sodium hydroxide is sold commercially. The qualifying word molten may be added in association with the proper shipping name when a hazardous material which is a solid in accordance with the definition in 1718 of this subchapter is offered for transportation in the molten state for example Alkylphenols solid nos molten. It is generally recommended that PVC is not used for air.
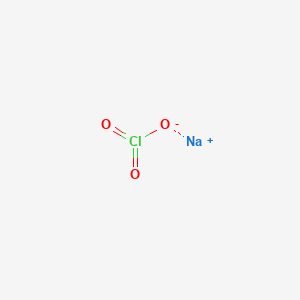
When molten however it can conduct electricity because its ions are able to move freely through the liquid Figure 3. Impurities like sulfur react with the oxygen to make SO2 gas which then escapes from the molten iron. Check the chemical compatibility of LDPE low density polyethylene with various chemicals solvents alcohols and other products. Sodium chloride is the salt most responsible. LDPE is defined by a density range of 09100940 gcm3.
 Source: chlorates.exrockets.com
Source: chlorates.exrockets.com
Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. Conductivity Only conducts electricity when Does not conduct Does conduct electricity molten or dissolved in water electricity all the in the solid state the the ionic bonds have broken valence electrons fourth valence electron and the ions are free to move. Contact may cause burns to skin eyes and mucous membranes. Transported at high temperatures. Bulk packagings must have exterior heating coils fusion welded to the tank shell which have been properly stress relieved.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title when does sodium chlorate become molten by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.