What is the solubility in water of benzoic acid
Home » chemical » What is the solubility in water of benzoic acid >What is the solubility in water of benzoic acid
What Is The Solubility In Water Of Benzoic Acid. A second oxygen atom dramatically increases water solubility as demonstrated by the compounds listed in the third row. Lipid solubility 13 determines the extent to which a drug partitions between an organic solvent and waterPropranolol oxprenolol metoprolol and timolol are the most lipid-soluble beta-adrenoceptor antagonists and atenolol nadolol and sotalol are the most water-soluble. Sulpiride remoxipride amisulpride tiapride sultopride veralipride aminohippuric acid cisapride imatinib and procainamide. It is the simplest amide derivative of benzoic acid.
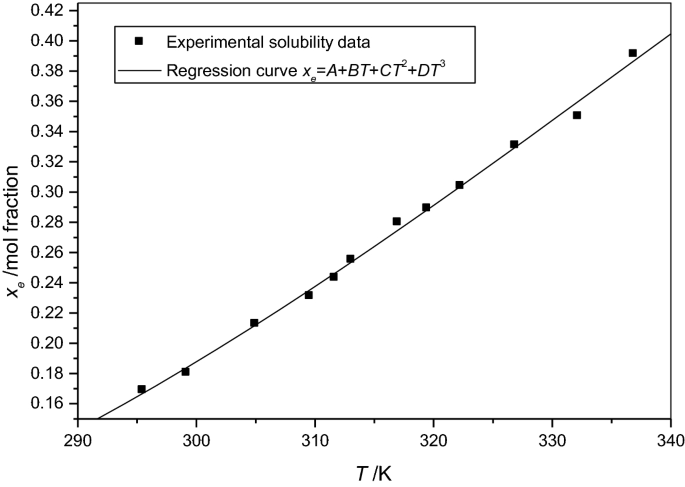 Determination And Correlation Of Solubilities Of Benzoic Acid Salicylic Acid Resorcinol And Hydroquinone In Water And In 1 Octanol At Temperatures From 297 25 K To 334 45 K Springerlink From link.springer.com
Determination And Correlation Of Solubilities Of Benzoic Acid Salicylic Acid Resorcinol And Hydroquinone In Water And In 1 Octanol At Temperatures From 297 25 K To 334 45 K Springerlink From link.springer.com
Benzoic Acid was recrystallized with a 41 recovery using 95 ethanol and water as the mixed-solvent. The free acid form is poorly soluble in water and the sodium salt sodium benzoate is often used because of its greater solubility Fig. In Meylers Side Effects of Drugs Sixteenth Edition 2016. A second oxygen atom dramatically increases water solubility as demonstrated by the compounds listed in the third row. It occurs naturally in prunes cinnamon and cloves. If a carboxylic acid ie benzoic acid was deprotonated using a base or an amine ie lidocaine was protonated using an acid it would become more water-soluble because the resulting specie carries a charge.
As we will learn when we study acid-base chemistry in a later chapter carboxylic acids such as benzoic acid are relatively weak acids and thus exist mostly in the acidic protonated form when added to pure water.
Again hydroxyl compounds are listed on the left. It is the simplest amide derivative of benzoic acid. Product loss by decarboxylation to benzoic acid is common. The poor solubility and low dissolution rate of poorly water soluble drugs in the aqueous gastrointestinal fluids often cause insufficient bioavailability. Benzoic acids antimicrobial activity is primarily against yeasts and. Chlorinated solvents ie dichloromethane chloroform exhibit a higher density than water while ethers hydrocarbons and many esters possess a lower density than water see.
 Source: researchgate.net
Source: researchgate.net
Atmospheric air can be used in its place but once reacted needs to be purified of toxins and ozone depleters such as methylbromide before being. Benzoic acids antimicrobial activity is primarily against yeasts and. 122 ºC the eutectic point is 82 ºC. Sulpiride remoxipride amisulpride tiapride sultopride veralipride aminohippuric acid cisapride imatinib and procainamide. The unknown sample may.
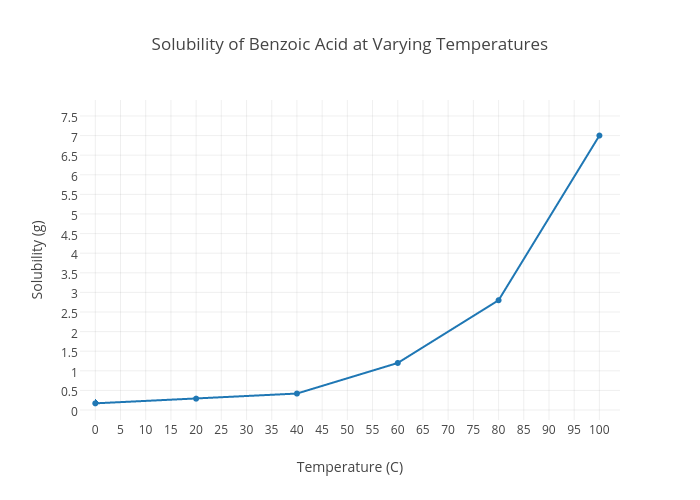 Source: chart-studio.plotly.com
Source: chart-studio.plotly.com
As for BCS. Product loss by decarboxylation to benzoic acid is common. Again hydroxyl compounds are listed on the left. The free acid form is poorly soluble in water and the sodium salt sodium benzoate is often used because of its greater solubility Fig. After extraction and purification the percent recovery and percentage composition of the unknown sample will be able to be calculated.
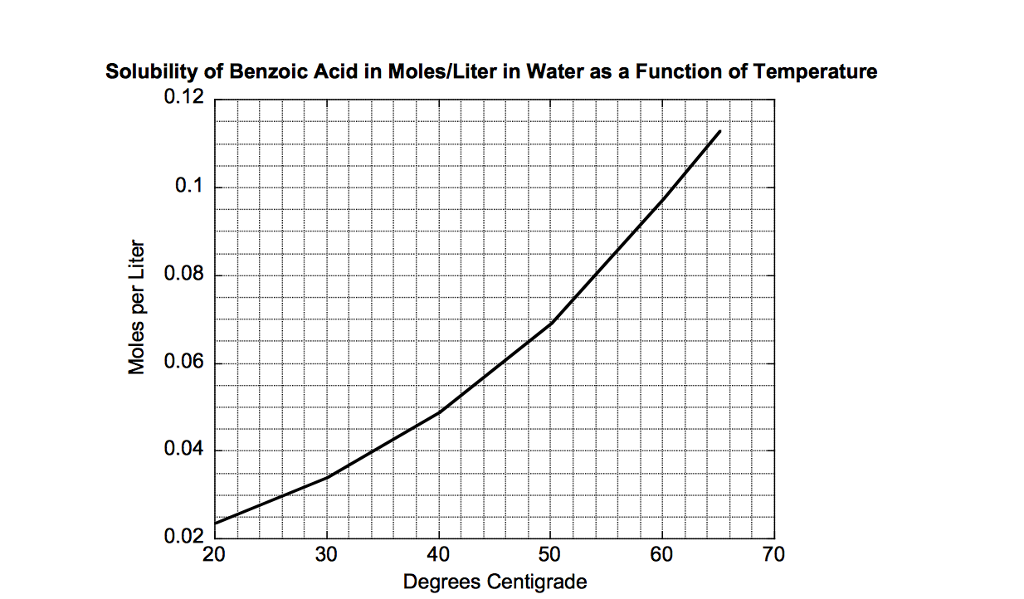 Source: chegg.com
Source: chegg.com
122 ºC the eutectic point is 82 ºC. In Meylers Side Effects of Drugs Sixteenth Edition 2016. Nitrogen exerts a solubilizing influence similar to oxygen as shown by the compounds in the fourth row. Atmospheric air can be used in its place but once reacted needs to be purified of toxins and ozone depleters such as methylbromide before being. They can be removed using vacuum filtration.
 Source: link.springer.com
Source: link.springer.com
Benzoic acid was also recrystallized with a 79 recovery using water as the solvent. In a chilled environment both compounds will not be soluble in water because the solubility of benzoic acid and 2-naphthol in water at 25C is 034g100mL and 0074g100mL respectively. Benzoic acid is an organic acid first used in foods almost 100 years ago. Acetic acid however is quite soluble. When a base is added the hydrogen ions from the weak acid break away and bond with the hydroxide ions to make water.
 Source: sciencedirect.com
Source: sciencedirect.com
Nitrogen exerts a solubilizing influence similar to oxygen as shown by the compounds in the fourth row. In Meylers Side Effects of Drugs Sixteenth Edition 2016. For example if A is cinnamic acid mp. As for BCS. Nitrogen exerts a solubilizing influence similar to oxygen as shown by the compounds in the fourth row.
 Source: ochemonline.wordpress.com
Source: ochemonline.wordpress.com
Nitrogen exerts a solubilizing influence similar to oxygen as shown by the compounds in the fourth row. In Meylers Side Effects of Drugs Sixteenth Edition 2016. Acebutolol and pindolol are. Benzoic acid is an organic acid first used in foods almost 100 years ago. When a base is added the hydrogen ions from the weak acid break away and bond with the hydroxide ions to make water.
 Source: researchgate.net
Source: researchgate.net
The high temperature diminishes oxygen solubility in an already oxygen-starved system. The high temperature diminishes oxygen solubility in an already oxygen-starved system. This is easy to explain using the small alcohol vs large alcohol argument. Chlorinated solvents ie dichloromethane chloroform exhibit a higher density than water while ethers hydrocarbons and many esters possess a lower density than water see. Lipid solubility 13 determines the extent to which a drug partitions between an organic solvent and waterPropranolol oxprenolol metoprolol and timolol are the most lipid-soluble beta-adrenoceptor antagonists and atenolol nadolol and sotalol are the most water-soluble.
 Source: degruyter.com
Source: degruyter.com
The free acid form is poorly soluble in water and the sodium salt sodium benzoate is often used because of its greater solubility Fig. Benzoic acid is an organic acid first used in foods almost 100 years ago. 122 ºC the eutectic point is 82 ºC. 137 ºC and B is benzoic acid mp. The poor solubility and low dissolution rate of poorly water soluble drugs in the aqueous gastrointestinal fluids often cause insufficient bioavailability.
 Source: degruyter.com
Source: degruyter.com
For example if A is cinnamic acid mp. For example if A is cinnamic acid mp. If a carboxylic acid ie benzoic acid was deprotonated using a base or an amine ie lidocaine was protonated using an acid it would become more water-soluble because the resulting specie carries a charge. As for BCS. The poor solubility and low dissolution rate of poorly water soluble drugs in the aqueous gastrointestinal fluids often cause insufficient bioavailability.
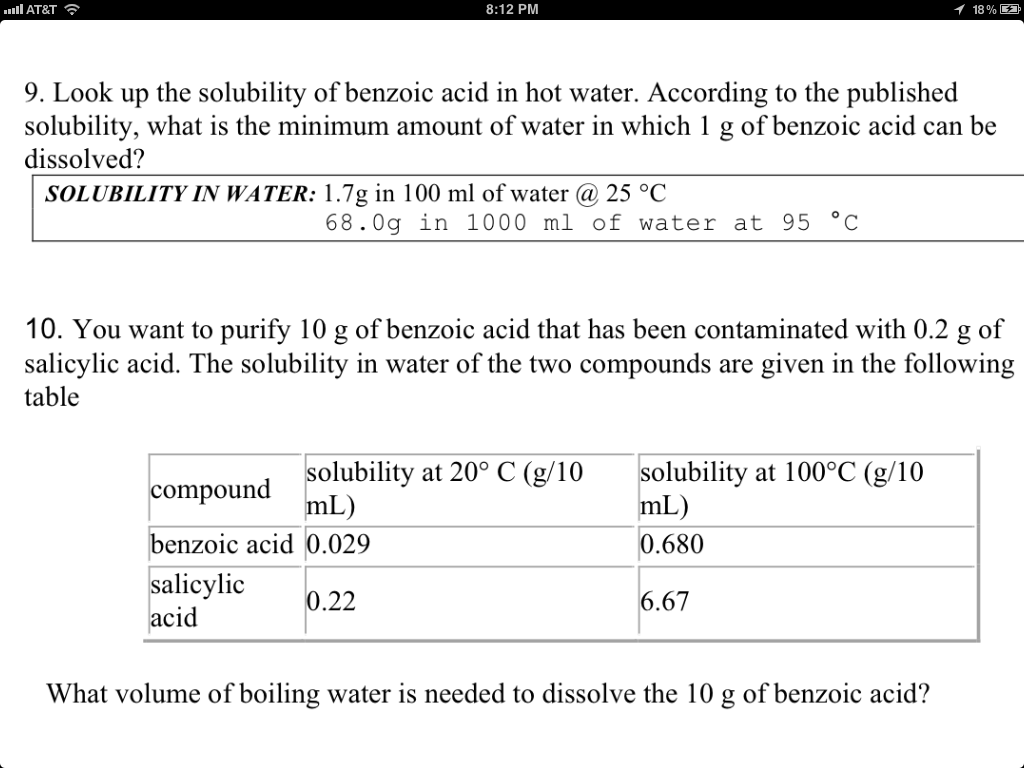 Source: chegg.com
Source: chegg.com
Sulpiride remoxipride amisulpride tiapride sultopride veralipride aminohippuric acid cisapride imatinib and procainamide. Benzoic acid is an organic acid first used in foods almost 100 years ago. It is slightly soluble in water and soluble in many organic solvents. Lipid solubility 13 determines the extent to which a drug partitions between an organic solvent and waterPropranolol oxprenolol metoprolol and timolol are the most lipid-soluble beta-adrenoceptor antagonists and atenolol nadolol and sotalol are the most water-soluble. Especially for class II low solubility and high permeability substances according to the BCS the bioavailability may be enhanced by increasing the solubility and dissolution rate of the drug in the gastro-intestinal fluids.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is the solubility in water of benzoic acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.