What does ethyl acetate
Home » chemical » What does ethyl acetate >What does ethyl acetate
What Does Ethyl Acetate. Hexa- hecta and hepta-esters of sucrose. Hydrogenated oil or fat artificial trans fats hydrogenated starch hydrolysate. Solvents are pressed out by hand followed by a. It is concluded that hydrolysis of ethyl acetate reaction in alkaline medium is a shifting order and due to its exothermic nature low reaction temperature promotes high conversion and reaction.

Like other lead compounds lead acetate and lead sulfide are toxic Selwyn 2005 although there is so little lead acetate in these papers that some safety data sheets do not label the papers as hazardous. ENGINEERING CONTROLS The level of protection and types of controls necessary will vary de pending upon potential exposure. I - J - K. Hexa- hecta and hepta-esters of sucrose. Iii aldehydes expressed in acetaldehyde. It is used as a solvent a synthetic flavoring substance and in making perfumes and dyes.
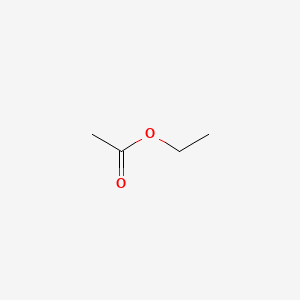
Esters expressed in ethyl acetate.
Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70 sulfuric acid Chem. Ethylenediamine tetraacetic acid EDTA. Ethyl acetate is perceived as the odour of nail polish remover and has a reported sensory threshold of 12 mgL. It is concluded that hydrolysis of ethyl acetate reaction in alkaline medium is a shifting order and due to its exothermic nature low reaction temperature promotes high conversion and reaction. This chemical is on the Special Health Hazard Substance List because it is. BP1125-4 CAS-No 141-78-6 Synonyms Acetic acid ethyl ester Recommended Use Laboratory chemicals.

Ethyl acetate is perceived as the odour of nail polish remover and has a reported sensory threshold of 12 mgL. Solvents are pressed out by hand followed by a. Now that weve got a basic framework to work with lets have a closer look at the before mentioned methods. Ethylenediamine tetraacetic acid EDTA. Ethyl acetate G F G F Ethyl alcohol VG VG VG VG Ethyl ether VG G VG G Ethylene dichloride F P F P Ethylene glycol VG VG VG VG Formaldehyde VG VG VG VG Chemical Neoprene Natural Latex or Rubber Butyl Nitrile Formic acid VG VG VG VG Freon 11 G P F G Freon 12 G P F G.
 Source: study.com
Source: study.com
15 grams per hectolitre of 100. 05 grams per hectolitre of 100 vol. Ethyl acetate is produced commercially from ethyl alcohol and acetic acid which in turn may be produced from natural ingredients or petroleum derivatives. It can be synthesized by reacting ethanol and butyric acid. Esters expressed in ethyl acetate.
 Source: acs.org
Source: acs.org
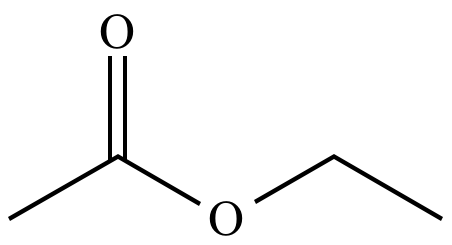
Ethyl acetate is perceived as the odour of nail polish remover and has a reported sensory threshold of 12 mgL. Ethyl acetate is produced commercially from ethyl alcohol and acetic acid which in turn may be produced from natural ingredients or petroleum derivatives. It can be synthesized by reacting ethanol and butyric acid. Flavouring ingredient Ethyl butyrate also known as ethyl butanoate or butyric ether is an ester with the chemical formula CH3CH2CH2COOCH2CH3 with one oxygen having a double bond. Product Name Ethyl acetate Cat No.
05 grams per hectolitre of 100 vol. It is soluble in propylene glycol paraffin oil and kerosene. Ethylene-vinyl acetate EVA also known as poly ethylene-vinyl acetate PEVA is the copolymer of ethylene and vinyl acetateThe weight percent of vinyl acetate usually varies from 10 to 40 with the remainder being ethylene. High fructose corn syrup. 13 grams per hectolitre of 100 vol.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Ethyl cyanoacetate is the ethyl ester of cyanoacetic acidEthyl cyanoacetate hydrolyzes rapidly under neutral and alkaline conditions to cyanoacetic acid and ethanol and so it does under most physiological and environmental conditions while in acid pH the half life is considerably longer. Hexa- hecta and hepta-esters of sucrose. These warnings inform Californians about their exposures to chemicals that cause cancer birth defects or other reproductive harm. How Does Extraction Compare To Distillation. Uses advised against Not for food drug pesticide or biocidal product use Details of the supplier of the safety data sheet Emergency Telephone Number CHEMTRECÒ Inside the USA.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The concentration of ethyl acetate ranges from about 30 60 mgL in normal wines to about 150 200 mgL in defective wines. Ethyl acetate is the major ester produced by yeast and at low levels can contribute fruity aroma properties and add complexity to wine. The results show that hydrolysis of the ethyl acetate is a forward one-way second order reaction. Where Does Cocaine Come From. REASON FOR CITATION Ethyl Acetate is on the Hazardous Substance List because it is regulated by OSHA and cited by ACGIH DOT NIOSH HHAG NFPA and EPA.
 Source: tcichemicals.com
Source: tcichemicals.com
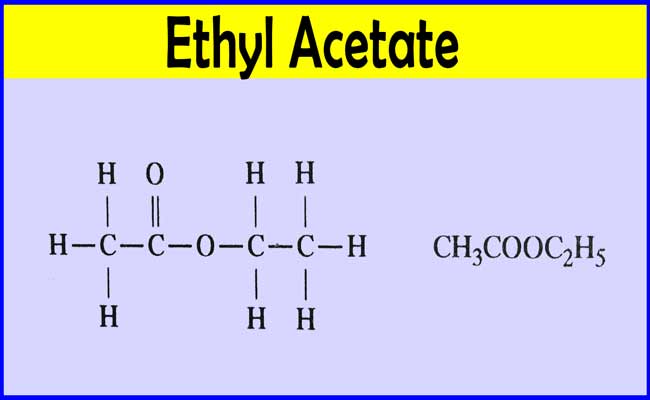
Ethyl Acetate is a colorless liquid with a fragrant fruity odor. Present in many fruits eg. Lead acetate is also known as sugar of lead and was used historically to sweeten and inadvertently poison wine Nriagu 1992. Welcome to the Proposition 65 Warnings Website. It is concluded that hydrolysis of ethyl acetate reaction in alkaline medium is a shifting order and due to its exothermic nature low reaction temperature promotes high conversion and reaction.
 Source: chem.ucla.edu
Source: chem.ucla.edu
The concentration of ethyl acetate ranges from about 30 60 mgL in normal wines to about 150 200 mgL in defective wines. Ethyl acetate G F G F Ethyl alcohol VG VG VG VG Ethyl ether VG G VG G Ethylene dichloride F P F P Ethylene glycol VG VG VG VG Formaldehyde VG VG VG VG Chemical Neoprene Natural Latex or Rubber Butyl Nitrile Formic acid VG VG VG VG Freon 11 G P F G Freon 12 G P F G. Uses advised against Not for food drug pesticide or biocidal product use Details of the supplier of the safety data sheet Emergency Telephone Number CHEMTRECÒ Inside the USA. It is soluble in propylene glycol paraffin oil and kerosene. Lead acetate is also known as sugar of lead and was used historically to sweeten and inadvertently poison wine Nriagu 1992.
 Source: chemistrypage.in
Source: chemistrypage.in
These warnings inform Californians about their exposures to chemicals that cause cancer birth defects or other reproductive harm. Ethyl acetate is perceived as the odour of nail polish remover and has a reported sensory threshold of 12 mgL. Ethyl acetate is produced commercially from ethyl alcohol and acetic acid which in turn may be produced from natural ingredients or petroleum derivatives. It can be synthesized by reacting ethanol and butyric acid. GMP disodium guanylate gold leaf.

Ethyl acetate is perceived as the odour of nail polish remover and has a reported sensory threshold of 12 mgL. Ethyl phenylacetate does not contain chromophores that absorb at wavelengths 290 nm and therefore is not expected to be susceptible to direct photolysis by sunlight. These warnings inform Californians about their exposures to chemicals that cause cancer birth defects or other reproductive harm. Alternately the base can be dissolved in a solvent such as acetone ether or ethyl acetate and heated in a bath of hot water. Like other lead compounds lead acetate and lead sulfide are toxic Selwyn 2005 although there is so little lead acetate in these papers that some safety data sheets do not label the papers as hazardous.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what does ethyl acetate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.