What does cobalt chloride paper test for
Home » chemical » What does cobalt chloride paper test for >What does cobalt chloride paper test for
What Does Cobalt Chloride Paper Test For. A Which row of the table shows the typical properties of a transition metal. This is the students method. Quantitative fit testing using a non-hazardous test aerosol such as corn oil polyethylene glycol 400 PEG 400 di-2-ethyl hexyl sebacate DEHS or sodium chloride generated in a test chamber and employing instrumentation to quantify the fit of the respirator. In association with Nuffield Foundation.

Cobalt and chromium. Buffers work by neutralizing any added acid H ions or base OH- ions to maintain the moderate pH making them a weaker acid or base. A very commonly used example of an alkaline buffer solution is a mixture of ammonia and ammonium chloride solution. Venereal Disease Research Lab VDRL. The resultant cesium chloride solution is separated from the gangue by filtration. Our Water Testing Kits are easy-to-use affordable fast and provide accurate results for drinking water tap water in the field in remote areas on-site or during manufacturing.
Buffers work by neutralizing any added acid H ions or base OH- ions to maintain the moderate pH making them a weaker acid or base.
Alkaline decomposition is carried out by roasting the pollucite ore with either a calcium carbonate-calcium chloride mix at 800-900 C or with a sodium carbonate-sodium chloride mix at 600-800 C followed by a water leach of the roasted mass. PTFE Chemical Compatibility Chart. The best diagnostic test for treponema pallidum is. NOT compatible with certain alkali metals and fluorinating agents such as xenon difluoride and cobaltIII fluoride. Silver chloride is a chemical compound with the chemical formula Ag ClThis white crystalline solid is well known for its low solubility in water this behavior being reminiscent of the chlorides of Tl and Pb 2Upon illumination or heating silver chloride converts to silver and chlorine which is signaled by grey to black or purplish coloration to some samples. How do buffers work.

Silver chloride is a chemical compound with the chemical formula Ag ClThis white crystalline solid is well known for its low solubility in water this behavior being reminiscent of the chlorides of Tl and Pb 2Upon illumination or heating silver chloride converts to silver and chlorine which is signaled by grey to black or purplish coloration to some samples. In association with Nuffield Foundation. Review the chemical compatibility of Teflon and PTFE with various chemicals solvents alcohols and other products in the cart below. This is the students method. It is insoluble in water but dissolves readily in acids and ammonium hydroxide.
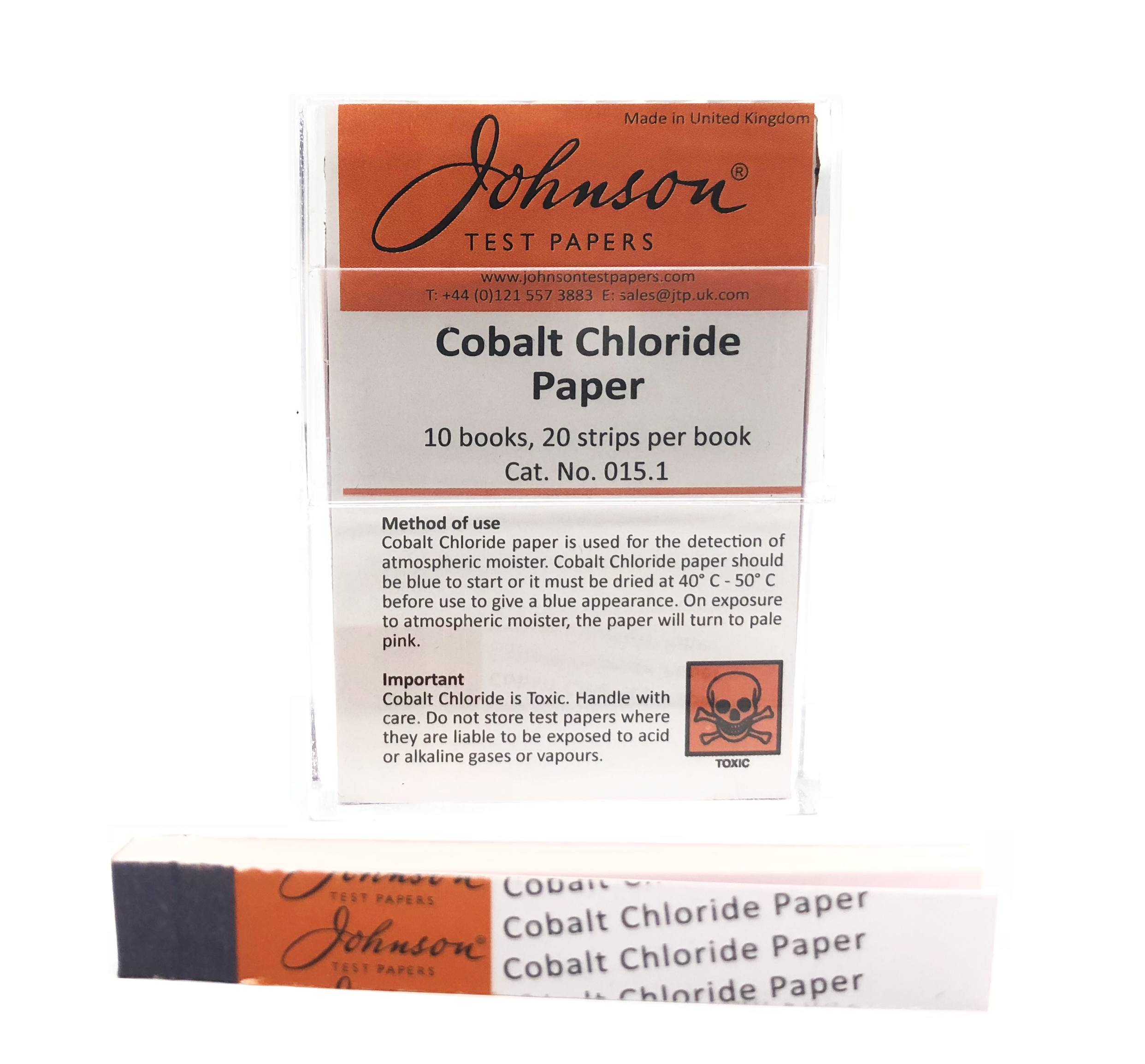 Source: philipharris.co.uk
Source: philipharris.co.uk
During incubation exclude all light to prevent the possibility of photosynthetic production of oxygen. The most common method of identification of Na is the flame test. He uses dilute hydrochloric acid and solid cobaltII oxide. This is the students method. When depleted in the body sodium must be replaced in order to maintain intracellular osmolarity nerve conduction muscle contraction and normal renal function.
 Source: chm.bris.ac.uk
Source: chm.bris.ac.uk
When depleted in the body sodium must be replaced in order to maintain intracellular osmolarity nerve conduction muscle contraction and normal renal function. 2 Mercuric nitrate 4500-Cl-C-2011. A very commonly used example of an alkaline buffer solution is a mixture of ammonia and ammonium chloride solution. A client visiting a family planning clinic is suspected of having an STI. The resultant cesium chloride solution is separated from the gangue by filtration.
 Source: preclaboratories.com
Source: preclaboratories.com
Cobalt and chromium. The resultant cesium chloride solution is separated from the gangue by filtration. Our water test kits are reliable and give complete peace of mind. 1 A anhydrous B aqueous C hydrated D saturated. Quantitative fit testing using a non-hazardous test aerosol such as corn oil polyethylene glycol 400 PEG 400 di-2-ethyl hexyl sebacate DEHS or sodium chloride generated in a test chamber and employing instrumentation to quantify the fit of the respirator.

Alkaline decomposition is carried out by roasting the pollucite ore with either a calcium carbonate-calcium chloride mix at 800-900 C or with a sodium carbonate-sodium chloride mix at 600-800 C followed by a water leach of the roasted mass. The first boiling tube contains cobalt chloride and the second contains limewater. He uses dilute hydrochloric acid and solid cobaltII oxide. Cobalt and chromium. NOT compatible with certain alkali metals and fluorinating agents such as xenon difluoride and cobaltIII fluoride.
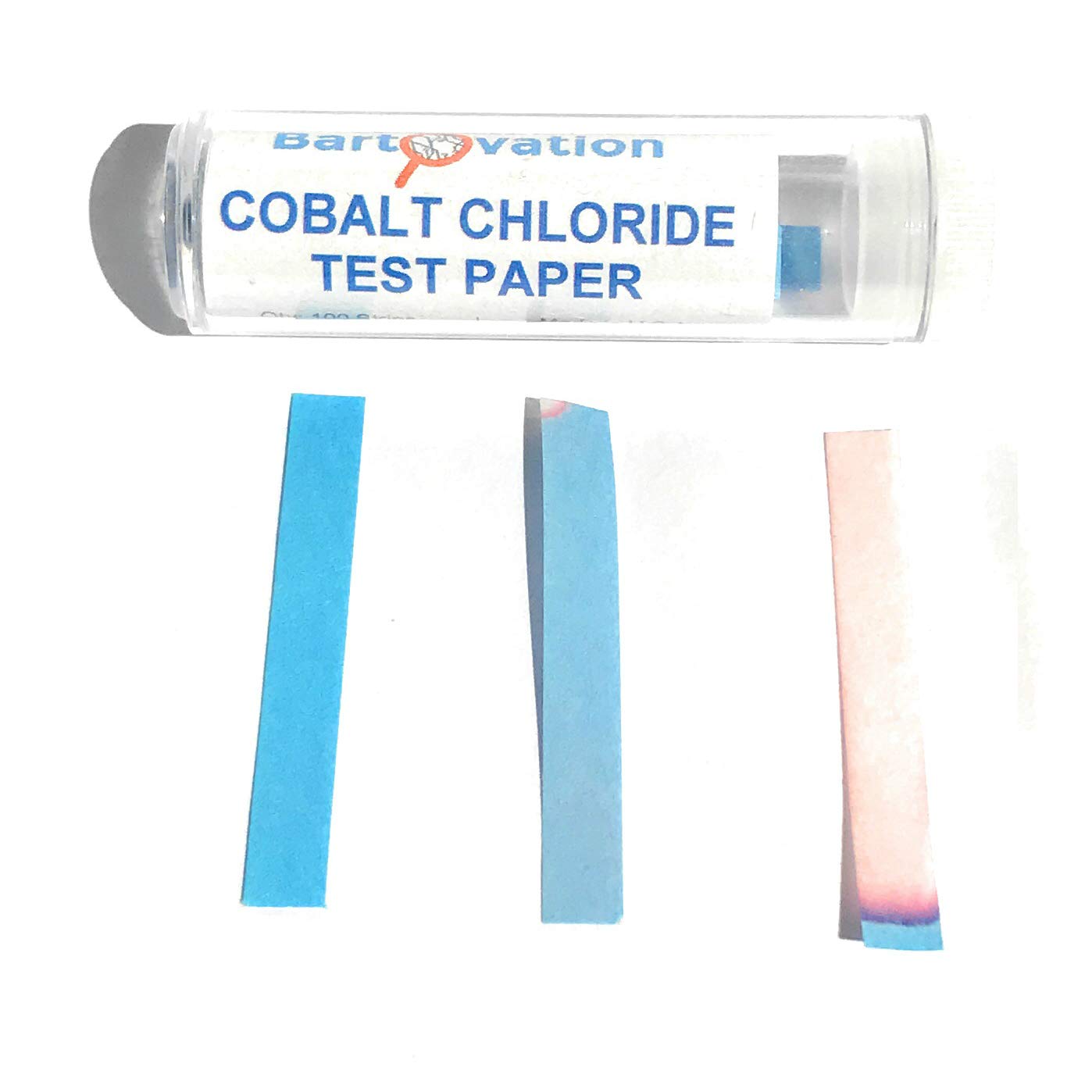 Source: amazon.co.uk
Source: amazon.co.uk
NH 4 aq OH-aq NH 3 g H 2 O. Make your own cobalt chloride indicator papers which can be used to test for the presence of water. The Electrochemical Society was founded in 1902 to advance the theory and practice at the forefront of electrochemical and solid state science and technology and allied subjects. Buffers work by neutralizing any added acid H ions or base OH- ions to maintain the moderate pH making them a weaker acid or base. NOT compatible with certain alkali metals and fluorinating agents such as xenon difluoride and cobaltIII fluoride.
 Source: slideplayer.com
Source: slideplayer.com
The best diagnostic test for treponema pallidum is. Step 1 pour about 50 cm3 of dilute hydrochloric acid into a beaker Step 2 warm the acid using a Bunsen burner Step 3 add a small amount of cobaltII oxide and stir the mixture with a glass rod Step 4 add further. Make your own cobalt chloride indicator papers which can be used to test for the presence of water. Alkaline decomposition is carried out by roasting the pollucite ore with either a calcium carbonate-calcium chloride mix at 800-900 C or with a sodium carbonate-sodium chloride mix at 600-800 C followed by a water leach of the roasted mass. This is the students method.

This is the students method. The most common method of identification of Na is the flame test. The first boiling tube contains cobalt chloride and the second contains limewater. Review the chemical compatibility of Teflon and PTFE with various chemicals solvents alcohols and other products in the cart below. It is insoluble in water but dissolves readily in acids and ammonium hydroxide.
 Source: sciencephoto.com
Source: sciencephoto.com
This test is very reliable. A Which row of the table shows the typical properties of a transition metal. NOT compatible with certain alkali metals and fluorinating agents such as xenon difluoride and cobaltIII fluoride. 2 Mercuric nitrate 4500-Cl-C-2011. Includes kit list and safety instructions.

A client visiting a family planning clinic is suspected of having an STI. I Which word describes both cobalt compounds in equation 1. 2 Mercuric nitrate 4500-Cl-C-2011. The compound reacts with the amino acid eccrine component of the fingerprint deposit to give a dark purple product known as Ruhemanns purple Figure 4The chemical processes involved are quite complex and development conditions such as temperature. The Electrochemical Society was founded in 1902 to advance the theory and practice at the forefront of electrochemical and solid state science and technology and allied subjects.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what does cobalt chloride paper test for by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.