Uses of hydrogen chloride
Home » chemical » Uses of hydrogen chloride >Uses of hydrogen chloride
Uses Of Hydrogen Chloride. Together with acid salts such as potassium hydrogen tartrate. Vinyl chloride is a gas at normal atmospheric temperature and pressure. In fact these uses of vinyl are often thought of as its only uses. Hydrogen bonding differs from other uses of the word bond since it is a force of attraction between a hydrogen atom in one molecule and a small atom of high electronegativity in another moleculeThat is it is an intermolecular force not an intramolecular force as in the common use of the word bond.
 Hydrochloric Acid Hcl Important Uses Applications Studiousguy From studiousguy.com
Hydrochloric Acid Hcl Important Uses Applications Studiousguy From studiousguy.com
Blood agents such as hydrogen cyanide or cyanogen chloride are designed to be delivered to the targeted area in the form of a vapour. It is used as a solvent to. HCl is an uncoloured gas and has a pungent aroma. This is a highly exothermic reaction and rarely used commercially to produce HCl. Ammonia hydrogen chloride ammonium chloride. Exposure to liquid hydrogen chloride may cause frostbite.
It is antagonistic to all muscle depressants no matter whether they act primarily on nerve or muscle.
It is used as a solvent to. Ammonia hydrogen chloride ammonium chloride. The Substance identity section is calculated from substance identification information from all ECHA databases. In making a freezing mixture which is used by icecream vendors. Vinyl chloride is a gas at normal atmospheric temperature and pressure. Upon contact with water it forms hydrochloric acid.
 Source: study.com
Source: study.com
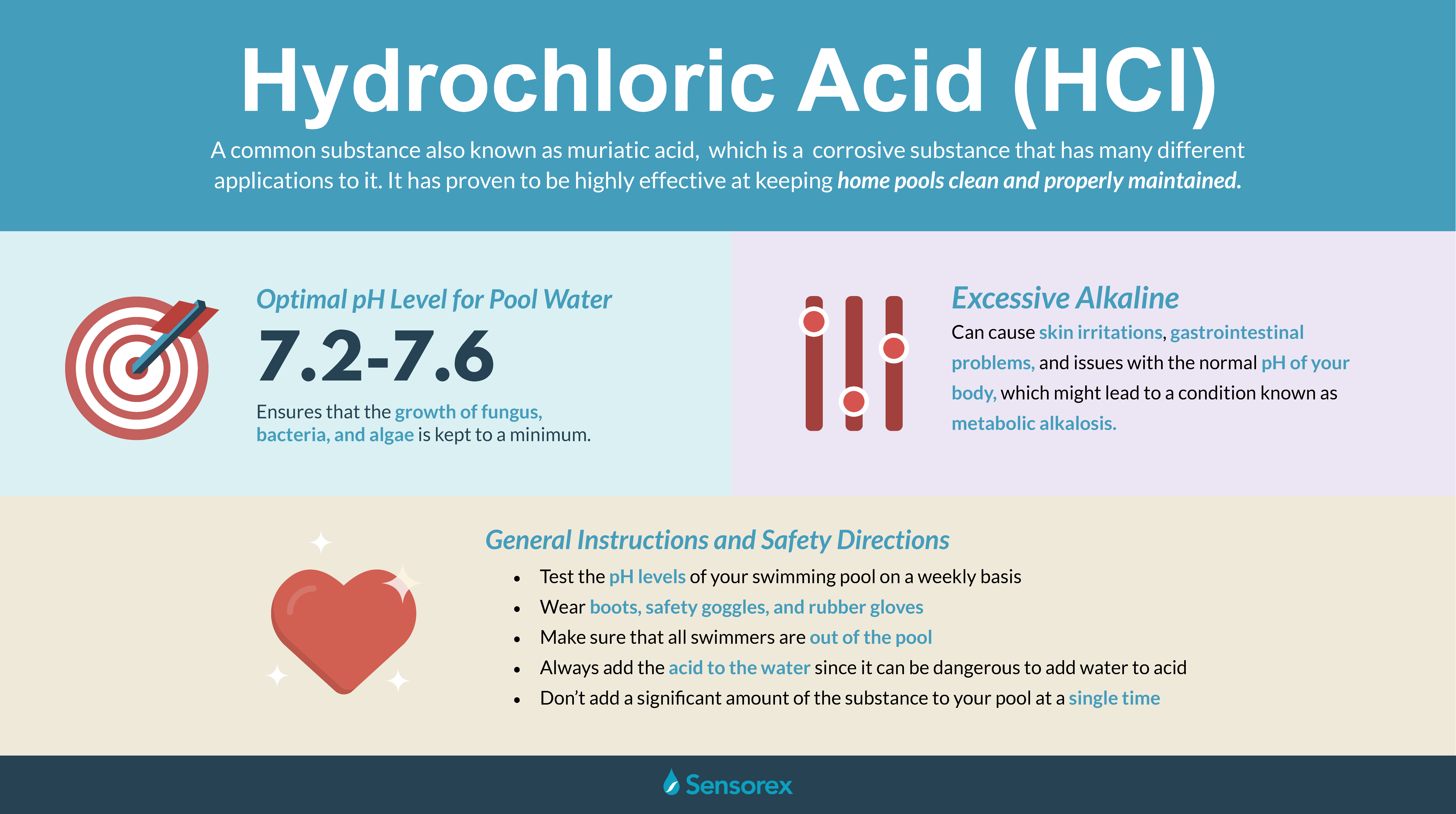
Hydrogen chloride has many uses including cleaning pickling electroplating metals tanning leather and refining and producing a wide variety of products. Magnesium gluconate may also be used for purposes. The Substance identity section is calculated from substance identification information from all ECHA databases. When we talk about the structure of AlCl3 it is sometimes. Hydrogen chloride can protonate molecules or ions and can also serve as an acid-catalyst for chemical reactions where anhydrous water-free conditions are desired.
 Source: sensorex.com
Source: sensorex.com
2Al 6HCl 2AlCl 3 H 2. NH 3 g HCl g NH 4 Cl s It typically takes just a few minutes for the ring to form but the exact time will depend on the dimensions of the tube the amount of the solutions which are put on the cotton wool wads and the temperature of the room. Hydrogen chloride can irritate the skin nose eyes throat and larynx. Workers may be harmed from exposure to hydrogen chloride. The substance identifiers displayed in the InfoCard are the best available substance name EC number CAS number andor the.
 Source: slideplayer.com
Source: slideplayer.com
The ring usually forms nearer to the hydrochloric acid end of the tube because hydrogen. Barium stimulates striated cardiac and smooth muscle regardless of innervation. In the preservation of food. The structure of solid DCl as determined by neutron diffraction of DCl powder at 77 K. Food industries for instance use hydrogen to make hydrogenated vegetable.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Magnesium gluconate is used as a supplement to treat low levels or to maintain adequate levels of this mineral in the body. Common Properties Of Vinyl. Sodium chloride NaCl An essential requirement of our food. CDC-ATSDR Toxic Substances Portal. Hydrogen cyanide or formonitrile HCN is a highly volatile and extremely.
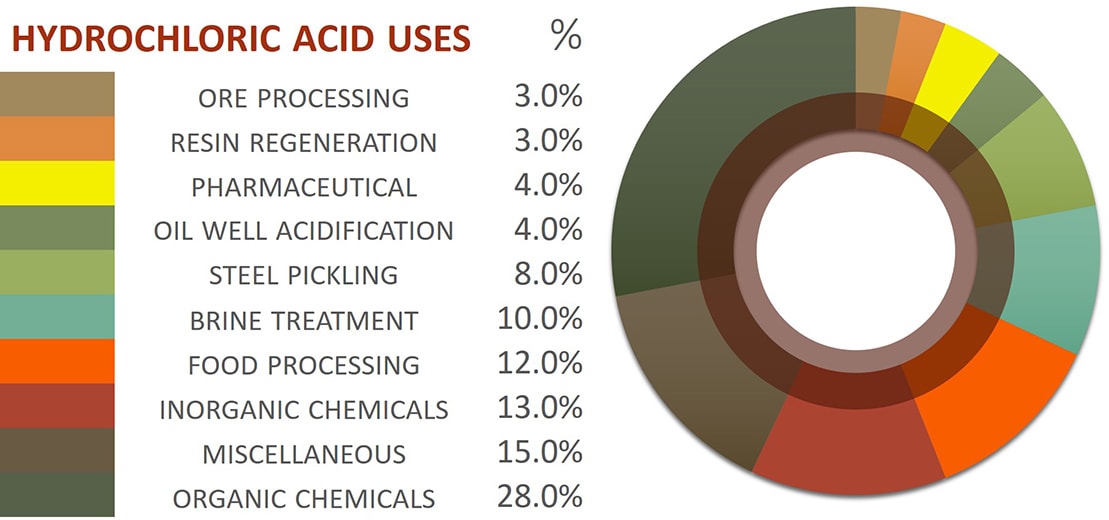 Source: protank.com
Source: protank.com
Substance identity Substance identity. Workers may be harmed from exposure to hydrogen chloride. Exposure to liquid hydrogen chloride may cause frostbite. It is used as a solvent to. The ring usually forms nearer to the hydrochloric acid end of the tube because hydrogen.
 Source: studiousguy.com
Source: studiousguy.com
The structure of solid DCl as determined by neutron diffraction of DCl powder at 77 K. NH 3 g HCl g NH 4 Cl s It typically takes just a few minutes for the ring to form but the exact time will depend on the dimensions of the tube the amount of the solutions which are put on the cotton wool wads and the temperature of the room. Initial stimulation of contraction leads to vasoconstriction through direct action on arterial muscle peristalsis through action on smooth muscle tremors and cramps through action on the skeletal muscles and. In the manufacture of soaps. Ammonia hydrogen chloride ammonium chloride.
 Source: studiousguy.com
Source: studiousguy.com
Both hydrogen chloride and hydrochloric acid are corrosive. Magnesium gluconate is used as a supplement to treat low levels or to maintain adequate levels of this mineral in the body. The Substance identity section is calculated from substance identification information from all ECHA databases. Hydrogen chloride can be formed during the burning of many plastics. Because of its acidic nature hydrogen chloride is a corrosive substance particularly in the presence of moisture.
 Source: chemistryworld.com
Source: chemistryworld.com
A buffer of carbonic acid H2CO3 and hydrogen carbonate HCO3- for example work in unison to keep the pH of the bloodstream at a neutral 74. It is flammable emitting hydrogen chloride. HCl is an uncoloured gas and has a pungent aroma. Hydrogen is used to turn unsaturated fats to saturated oils and fats. Together with acid salts such as potassium hydrogen tartrate.
 Source: medicinenet.com
Source: medicinenet.com
Substance identity Substance identity. Workers may be harmed from exposure to hydrogen chloride. Hydrochloric acid is the aqueous solution of hydrogen chloride. NH 3 g HCl g NH 4 Cl s It typically takes just a few minutes for the ring to form but the exact time will depend on the dimensions of the tube the amount of the solutions which are put on the cotton wool wads and the temperature of the room. Some common ways are by reacting the aluminium metal with hydrogen chloride or by conducting a single displacement reaction between copper chloride and aluminium metal.
 Source: slideplayer.com
Source: slideplayer.com
Hydrogen chloride has many uses including cleaning pickling electroplating metals tanning leather and refining and producing a wide variety of products. Common Properties Of Vinyl. HCl is an uncoloured gas and has a pungent aroma. Magnesium gluconate is used as a supplement to treat low levels or to maintain adequate levels of this mineral in the body. Buffer solutions have a wide array of uses not only within chemistry and biology labs but in everyday life as well.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title uses of hydrogen chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.