Tin ii oxide
Home » chemical » Tin ii oxide >Tin ii oxide
Tin Ii Oxide. K 2 Se - Potassium Selenide. It has tin in its 4 oxidation state. It is a white powdery solid. Tin wont reduce vanadiumIII to.
 Tin Ii Oxide American Elements From americanelements.com
Tin Ii Oxide American Elements From americanelements.com
TinIV oxide also known as tin dioxide or stannic oxide is a chemical compound. K 2 O - Potassium Oxide. It is a white powdery solid. Cs 2 O - Cesium Oxide. K 2 S - Potassium Sulfide. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A.
Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A.
TinII oxide stannous oxide is a compound with the formula SnO. It reacts with acids to produce stannic salts such as tinIV chloride with hydrochloric acid. Cs 2 O - Cesium Oxide. What is Infobox references. Na 2 S - Sodium Sulfide. BeCl 2 - Beryllium Chloride.

That works as well. Li 2 Se - Lithium Selenide. It is composed of tin and oxygen where tin has the oxidation state of 2. K 2 O - Potassium Oxide. Sc 2 S - Cesium Sulfide.

K 2 O - Potassium Oxide. SnSe - TinII Selenide. Na 2 S - Sodium Sulfide. Sc 2 S - Cesium Sulfide. It reacts with strong bases to make stannites.
 Source: americanelements.com
Source: americanelements.com
Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. There are two forms a stable blue-black form and a metastable red form. IC50 of 23 nM. It has tin and oxide ions in it. Tin wont reduce vanadiumIII to.
 Source: gelest.com
Source: gelest.com
Na 2 S - Sodium Oxide. Li 2 S - Lithium Sulfide. It is composed of tin and oxygen where tin has the oxidation state of 2. K 2 Se - Potassium Selenide. It reacts with strong bases to make stannites.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sc 2 S - Cesium Sulfide. Na 2 S - Sodium Sulfide. It is a white powdery solid. Cs 2 O - Cesium Oxide. Cs 2 Se - Cesium Selenide.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Na 2 S - Sodium Oxide. Stearoyl-CoA Desaturase 1 Inhibitor MF-438 CAS 921605-87-0 is a cell-permeable inhibitor of Stearoyl-CoA Desaturase 1 SCD1. It reacts with carbon at a. Tin wont reduce vanadiumIII to. In the first vanadium equation from 5 to 4 the tin value is more negative.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Tin wont reduce vanadiumIII to. IC50 of 23 nM. In the first vanadium equation from 5 to 4 the tin value is more negative. Li 2 S - Lithium Sulfide. But in the final vanadium reaction from 3 to 2 tin no longer has the more negative E value.
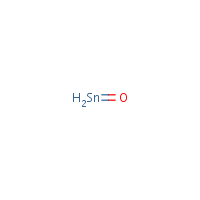 Source: haz-map.com
Source: haz-map.com
It reacts with carbon at a. But in the final vanadium reaction from 3 to 2 tin no longer has the more negative E value. In the second vanadium equation from 4 to 3 the tin value is again the more negative. Tin wont reduce vanadiumIII to. Na 2 S - Sodium Sulfide.
 Source: youtube.com
Source: youtube.com
Li 2 Se - Lithium Selenide. TinIV oxide also known as tin dioxide or stannic oxide is a chemical compound. Na 2 S - Sodium Sulfide. Cs 2 Se - Cesium Selenide. Li 2 Se - Lithium Selenide.
 Source: samaterials.com
Source: samaterials.com
In the second vanadium equation from 4 to 3 the tin value is again the more negative. It has tin and oxide ions in it. K 2 Se - Potassium Selenide. In the first vanadium equation from 5 to 4 the tin value is more negative. BeF 2 - Beryllium Fluoride.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title tin ii oxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.