Tin chloride molar mass
Home » chemical » Tin chloride molar mass >Tin chloride molar mass
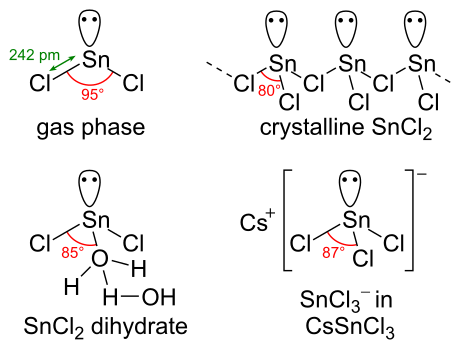
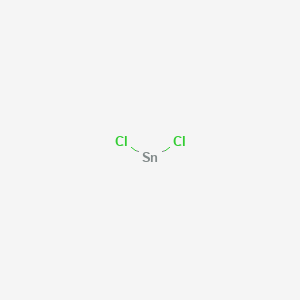
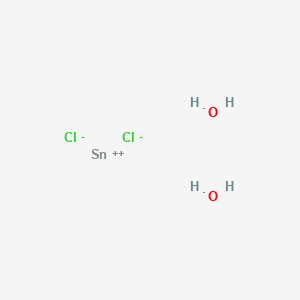
Tin Chloride Molar Mass. 1 u is equal to 112 the mass of one atom of carbon-12 Molar mass molar weight is the mass of one mole of a substance and is expressed in gmol. Referring to the periodic table the atomic mass of K is 3910 amu and so its molar mass is 3910 gmol. Sodium chloride is an ionic compound composed of sodium cations Na and chloride anions Cl combined in a 11 ratio. TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in.
 Tin Ii Chloride Wikipedia From en.wikipedia.org
Tin Ii Chloride Wikipedia From en.wikipedia.org
The formula mass for this compound is computed as 5844 amu see Figure 3. The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions. The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. 11556 gmol Appearance Hygroscopic white crystals Density. Grams Unit of moles.
The mass in grams of a compound is equal to its molarity in moles multiply its molar mass.
417 K Boiling point. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. For pure solids liquids and gases Unit of mass. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. 1 u is equal to 112 the mass of one atom of carbon-12 Molar mass molar weight is the mass of one mole of a substance and is expressed in gmol. Molar mass Mr for a compound can be calculated by adding up the mass numbers f rom the periodic table of each element in the compound eg CaCO3 401 120 160 x3 1001 1.
 Source: en.wikipedia.org
Source: en.wikipedia.org
022045 x 1023 particles One mole of common Substances CaCO3 100. Referring to the periodic table the atomic mass of K is 3910 amu and so its molar mass is 3910 gmol. 417 K Boiling point. For pure solids liquids and gases Unit of mass. Molar mass Mr for a compound can be calculated by adding up the mass numbers f rom the periodic table of each element in the compound eg CaCO3 401 120 160 x3 1001 1.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Grams mole molar mass. The given mass of K 47 g is a bit more than one-tenth the molar mass 3910 g so a reasonable ballpark estimate of the number of moles would be slightly greater than 01 mol. Sodium chloride is an ionic compound composed of sodium cations Na and chloride anions Cl combined in a 11 ratio. Grams Unit of moles.

Sodium chloride is an ionic compound composed of sodium cations Na and chloride anions Cl combined in a 11 ratio. 11556 gmol Appearance Hygroscopic white crystals Density. Referring to the periodic table the atomic mass of K is 3910 amu and so its molar mass is 3910 gmol. The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions. Grams Unit of moles.

Sodium chloride is an ionic compound composed of sodium cations Na and chloride anions Cl combined in a 11 ratio. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm. One formula unit of sodium chloride. Decomposes Solubility in water. 1 0 C Acidity pK a 5 Hazards Main hazards.
 Source: cs.mcgill.ca
Source: cs.mcgill.ca
Grams Unit of moles. Referring to the periodic table the atomic mass of K is 3910 amu and so its molar mass is 3910 gmol. Soluble in chloroform ethanol insoluble in diethyl ether. The same concept can be extended to ionic compounds and molecules. The formula mass for this compound is computed as 5844 amu see Figure 3.
1 0 C Acidity pK a 5 Hazards Main hazards. 07 g SO 2 SO 2 23. For pure solids liquids and gases Unit of mass. Calculate the molecular mass of potassium permanganate KMnO4. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams.
Grams 58443 5 292215 g. The atomic mass is useful in chemistry when it is paired with the mole concept. The same concept can be extended to ionic compounds and molecules. 11556 gmol Appearance Hygroscopic white crystals Density. Table salt NaCl contains an array of sodium and chloride ions combined in a 11 ratio.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. Grams 58443 5 292215 g. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. The given mass of K 47 g is a bit more than one-tenth the molar mass 3910 g so a reasonable ballpark estimate of the number of moles would be slightly greater than 01 mol. 1 u is equal to 112 the mass of one atom of carbon-12 Molar mass molar weight is the mass of one mole of a substance and is expressed in gmol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Calculate the molecular mass of potassium permanganate KMnO4. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Sodium chloride is an ionic compound composed of sodium cations Na and chloride anions Cl combined in a 11 ratio. Table salt NaCl contains an array of sodium and chloride ions combined in a 11 ratio. Referring to the periodic table the atomic mass of K is 3910 amu and so its molar mass is 3910 gmol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
417 K Boiling point. Sodium chloride is an ionic compound composed of sodium cations Na and chloride anions Cl combined in a 11 ratio. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Grams 58443 5 292215 g. Irritant Safety data sheet.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title tin chloride molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.