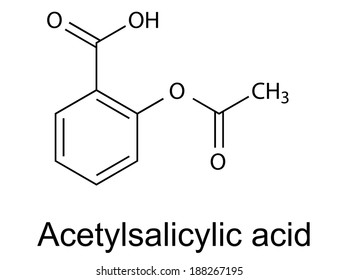
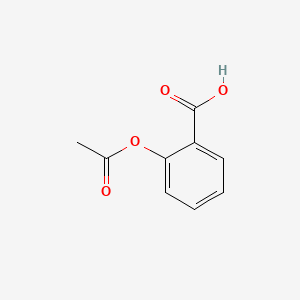
Structure of acetylsalicylic acid
Home » chemical » Structure of acetylsalicylic acid >Structure of acetylsalicylic acid
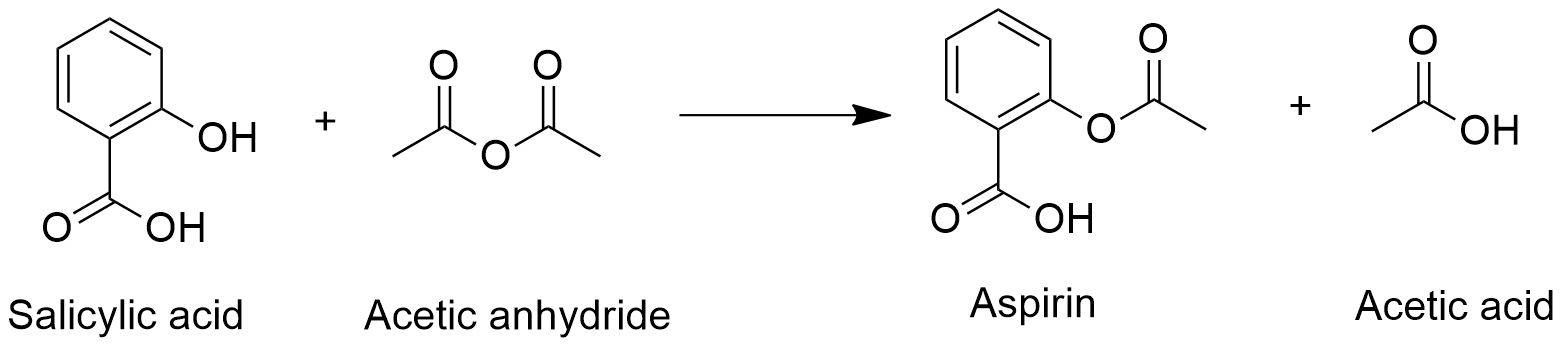
Structure Of Acetylsalicylic Acid. It is a non-steroidal anti-inflammatory drug. Whereas other scientists had focused on the carboxylic acid group Dr Felix Hoffman a German chemist at Friedrich Bayer and Co concentrated on the phenol group and managed on August 10 th 1897 to acetylate the phenol group and produce pure stable acetylsalicylic acid ASA for the first time 12. Aspirin is one of the safest and most effective medicines in the world. Since its related to aspirin acetylsalicylic acid it also has anti-inflammatory properties.
 The Structural Formula Of Acetylsalicylic Acid Download Scientific Diagram From researchgate.net
The Structural Formula Of Acetylsalicylic Acid Download Scientific Diagram From researchgate.net
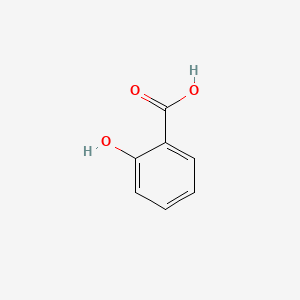
It consists of a hydroxyl group -OH group attached to the orthro in relation to the carboxylic acid group -COOH group present in the benzene ring. Aspirin also known as acetylsalicylic acid ASA is a medication used to reduce pain fever or inflammation. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease pericarditis and rheumatic fever. Share on Pinterest. Salicylic acid HOC6H4COOH or C7H6O3 CID 338 - structure chemical names physical and chemical properties classification patents literature biological. The second chapter focuses on.
Acetylsalicylic acid Recruiting Phase 0 Trials for Malignant Peritoneal Neoplasm Fallopian Tubes Cancer Ovarian Cancer Treatment Back to Acetylsalicylic acid Indications.
Aspirin is a salicylate drug often used as an analgesic to relieve minor aches and pains as an anti-inflammatory compound that inhibits Cox-1. Also known as Aspirin acetylsalicylic acid ASA is a commonly used drug for the treatment of pain and fever due to various causesAcetylsalicylic acid has both anti-inflammatory and antipyretic effects. Although salicylic acid was effective at reducing pain and fever it also had some unpleasant side effects. Image will be uploaded soon. This drug also inhibits platelet aggregation and is used in the prevention of blood. In reaction 1 a base eg sodium.
Aspirin USAN also known as acetylsalicylic acid is a salicylate drug often used as ananalgesic to relieve minor aches and pains as an antipyretic to reduce fever and as an anti-inflammatory medication. Its soluble in water. Whereas other scientists had focused on the carboxylic acid group Dr Felix Hoffman a German chemist at Friedrich Bayer and Co concentrated on the phenol group and managed on August 10 th 1897 to acetylate the phenol group and produce pure stable acetylsalicylic acid ASA for the first time 12. It is a white crystalline solid at room temperature. The history of aspirin also known as acetylsalicylic acid begins with its synthesis and manufacture in 1899Before that salicylic acid had been used medicinally since antiquityMedicines made from willow and other salicylate-rich plants appear in clay tablets from ancient Sumer as well as the Ebers Papyrus from ancient Egypt.
 Source: shutterstock.com
Source: shutterstock.com
However while some studies have. Visit BYJUS to understand the Properties Structure Synthesis Uses Health risk and FAQs of Aspirin or Acetylsalicylic acid. Aspirin USAN also known as acetylsalicylic acid is a salicylate drug often used as ananalgesic to relieve minor aches and pains as an antipyretic to reduce fever and as an anti-inflammatory medication. The structure of aspirin is given below. Also known as Aspirin acetylsalicylic acid ASA is a commonly used drug for the treatment of pain and fever due to various causes.
 Source: softschools.com
Source: softschools.com
Its boiling point is 140. Visit BYJUS to understand the Properties Structure Synthesis Uses Health risk and FAQs of Aspirin or Acetylsalicylic acid. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease pericarditis and rheumatic fever. The structure of aspirin is given below. Its IUPAC name is 2-hydroxybenzoic acid.
 Source: toppr.com
Source: toppr.com
Although salicylic acid was effective at reducing pain and fever it also had some unpleasant side effects. Due to its physical properties such as an acid taste without any corrosive action acetylsalicylic acid differs advantageously from salicylic acid and is being examined for its usefulness with just this in mind The German is however grammatically awkward and the sentence is capable of being misread to mean that the compound was about to be tested rather. Its IUPAC name is 2-hydroxybenzoic acid. The history of aspirin also known as acetylsalicylic acid begins with its synthesis and manufacture in 1899Before that salicylic acid had been used medicinally since antiquityMedicines made from willow and other salicylate-rich plants appear in clay tablets from ancient Sumer as well as the Ebers Papyrus from ancient Egypt. The structure of salicylic acid is shown below.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The history of aspirin also known as acetylsalicylic acid begins with its synthesis and manufacture in 1899Before that salicylic acid had been used medicinally since antiquityMedicines made from willow and other salicylate-rich plants appear in clay tablets from ancient Sumer as well as the Ebers Papyrus from ancient Egypt. C10B Lipid modifying agents combinations. Visit BYJUS to understand the Properties Structure Synthesis Uses Health risk and FAQs of Aspirin or Acetylsalicylic acid. Aspirin USAN also known as acetylsalicylic acid is a salicylate drug often used as ananalgesic to relieve minor aches and pains as an antipyretic to reduce fever and as an anti-inflammatory medication. Acetylsalicylic acid commonly known as aspirin absorbs light in the UV region of the electromagnetic spectrum.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Structure of Acetylsalicylic Acid. The structure of salicylic acid is shown below. 3g of aspirin can dissolve in 1 liter of water. Acetylsalicylic acid Commonly known or available as Aspirin DrugBank Accession Number DB00945 Background. This drug also inhibits platelet aggregation and is used in the prevention of blood.
 Source: researchgate.net
Source: researchgate.net
Navaratnam K Alfirevic Z Pirmohamed M Alfirevic A. Aspirin acetylsalicylic acid is an aromatic compound containing both a carboxylic acid functional group and an ester functional group. 3g of aspirin can dissolve in 1 liter of water. B01AC06 acetylsalicylic acid B01AC07 dipyridamole B01AC30 combinations eg. Acetylsalicylic acid Recruiting Phase 0 Trials for Malignant Peritoneal Neoplasm Fallopian Tubes Cancer Ovarian Cancer Treatment Back to Acetylsalicylic acid Indications.
 Source: researchgate.net
Source: researchgate.net
Preparation of aspirin and Acetylsalicylic acid structureaspirin. However while some studies have. Aspirin Acetylsalicylic acid- C9H8O4 - Acetylsalicylic Acid also called aspirin is one of the most widely used nonprescription drugs. Acetylsalicylic acid commonly known as aspirin absorbs light in the UV region of the electromagnetic spectrum. It is also used for blood clots heart attacks strokes and bowel cancer prevention.
 Source: researchgate.net
Source: researchgate.net
Aspirin USAN also known as acetylsalicylic acid is a salicylate drug often used as ananalgesic to relieve minor aches and pains as an antipyretic to reduce fever and as an anti-inflammatory medication. Its IUPAC name is 2-hydroxybenzoic acid. Navaratnam K Alfirevic Z Pirmohamed M Alfirevic A. Its soluble in water. Due to its physical properties such as an acid taste without any corrosive action acetylsalicylic acid differs advantageously from salicylic acid and is being examined for its usefulness with just this in mind The German is however grammatically awkward and the sentence is capable of being misread to mean that the compound was about to be tested rather.
Update and literature review. In reaction 1 a base eg sodium. Therefore we must perform a series of chemical reactions to convert acetylsalicylic acid to a colored complex as shown in Figure 5. Whereas other scientists had focused on the carboxylic acid group Dr Felix Hoffman a German chemist at Friedrich Bayer and Co concentrated on the phenol group and managed on August 10 th 1897 to acetylate the phenol group and produce pure stable acetylsalicylic acid ASA for the first time 12. Its boiling point is 140.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title structure of acetylsalicylic acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.