Strontium sulfate solubility
Home » chemical » Strontium sulfate solubility >Strontium sulfate solubility
Strontium Sulfate Solubility. K s Sr 2 SO 4 2 S 010 S 28. Method 3 of 3. CH 4 N 2 S. Memorizing the Basics.

Table 1 Typical Chemical Constituents of Concern. 710 gL 18 C 660 gL 20 C tetrahydrate. 6043 gL 0 C 2065 gL 100 C Solubility. These 3 ions are never soluble with sulfates. Tl 2 CrO 4. CH 4 N 2 S.
Ionic Compound Formula K sp.
Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent to form a solution of the solute in the solvent. Table 1 Typical Chemical Constituents of Concern. H 2 SO 4. The sulfate ion generally forms soluble compounds but there are several exceptions. An equilibrium constant is the ratio of the concentration of the products of a reaction divided by the concentration of the reactants once the reaction has reached equilibrium. Solubility functions by a group of rules that determine how dissolvable a substance solute is in solvent and depends entirely on the physical and chemical properties of the solute and solvent.
 Source: youtube.com
Source: youtube.com
Only two minerals celestite strontium sulfate and strontianite strontium carbonate however contain strontium in sufficient quantities to make its recovery practical. Sulfate and strontium chloride solution. Orally admin strontium nitrate accumulated in skeleton of rats in proportion to dose as determined in 4- 8-wk feeding tests. Sulfate reduction to sulfide generally accompanies the precipitation of pyrite iron sulfide cinnabar mercury sulfide galena lead sulfide and many more minerals. Artificial bone can be created from ceramics such as calcium phosphates eg HA and tricalcium phosphate bioglass and calcium sulphate are biologically active depending on solubility in physiological environment.
 Source: sciencecoverage.com
Source: sciencecoverage.com
H 2 SO 4. These 3 ions are never soluble with sulfates. Add aqueous strontium chloride SrCl 2 solution to the sulfate ion solution and observe the changes. Sulfate and strontium chloride solution. The solubility product constant K sp is the equilibrium constant for a solid that dissolves in an aqueous solutionAll of the rules for determining equilibrium constants continue to apply.
 Source: solutioninn.com
Source: solutioninn.com
That is solutes typically will dissolve best in solvents that have the most molecular similarities. All salts of sulfides and. All oxides and hydroxides are insoluble except for those of group IA calcium strontium and barium. Artificial bone can be created from ceramics such as calcium phosphates eg HA and tricalcium phosphate bioglass and calcium sulphate are biologically active depending on solubility in physiological environment. Strontium is the chemical element with the symbol Sr and atomic number 38.

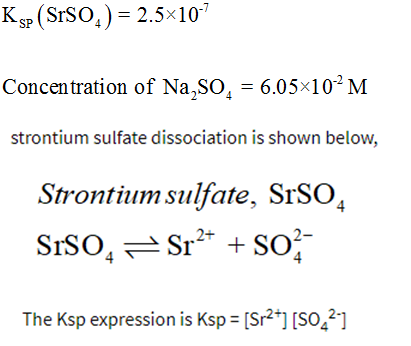
Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3. Strontium Sr 2 barium Ba 2 lead Pb 2 silver Ag calcium Ca 2 radium Ra 2 and diatomic silver Ag 2 2. H 2 C 4 H 4 O 6. S K s 28 10 7 ½ 53 10 4 b In 010 mol L 1 Na 2 SO 4 we have. The metal forms a dark oxide layer when it is exposed to air.
 Source: chem.fsu.edu
Source: chem.fsu.edu
Castro Bear may sound silly but it stands for the 3 ions Calcium Ca 2 Strontium Sr 2 and Barium Ba 2. Soluble in ammonia very slightly soluble in ethanol acetone insoluble in nitric acid. These 3 ions are never soluble with sulfates. The amikacin sulfate injection is indicated in the short-term treatment of serious bacterial infections due to susceptible strains of gram-negative bacteria including Pseudomonas species Escherichia coli species of indole-positive and indole-negative Proteus Providencia species Klebsiella-Enterobacter-Serratia species as well as Acinetobacter Mima-Herellea species. One may also speak of solid solution but rarely of solution in a gas.
 Source: scialert.net
Source: scialert.net
6043 gL 0 C 2065 gL 100 C Solubility. 6043 gL 0 C 2065 gL 100 C Solubility. Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent to form a solution of the solute in the solvent. Emission of gases Identify ammonium salts identify d block cations by ammonia solution Solubility if inorganic Compounds spd block elements Identify halides Identify carbonate ions identify chromium compounds Testing for thiosulfate S 2 O 3 2. When writing the mnemonic put a cross next to Castro Bear and another cross next to the S in SAG to remember that these ions are exceptions to the sulfate solubility.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The metal forms a dark oxide layer when it is exposed to air. Castro Bear may sound silly but it stands for the 3 ions Calcium Ca 2 Strontium Sr 2 and Barium Ba 2. H 2 C 4 H 4 O 6. All salts of sulfate are soluble except for barium sulfate leadII sulfate and strontium sulfate. Industry is one place.

Artificial bone can be created from ceramics such as calcium phosphates eg HA and tricalcium phosphate bioglass and calcium sulphate are biologically active depending on solubility in physiological environment. Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent to form a solution of the solute in the solvent. These 3 ions are never soluble with sulfates. Orally admin strontium nitrate accumulated in skeleton of rats in proportion to dose as determined in 4- 8-wk feeding tests. Strontium is the chemical element with the symbol Sr and atomic number 38.
 Source: bartleby.com
Source: bartleby.com
All salts of sulfate are soluble except for barium sulfate leadII sulfate and strontium sulfate. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3. Most often the solvent is a liquid which can be a pure substance or a mixture. Industry is one place. An equilibrium constant is the ratio of the concentration of the products of a reaction divided by the concentration of the reactants once the reaction has reached equilibrium.
 Source: youtube.com
Source: youtube.com
An alkaline earth metal strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. Artificial bone can be created from ceramics such as calcium phosphates eg HA and tricalcium phosphate bioglass and calcium sulphate are biologically active depending on solubility in physiological environment. All oxides and hydroxides are insoluble except for those of group IA calcium strontium and barium. Magnetic susceptibility χ 57210 6 cm 3 mol Structure Crystal structure. These materials combine with growth factors ions such as strontium or mixed with bone marrow aspirate to increase biological activity.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title strontium sulfate solubility by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.