Solubility of no2
Home » chemical » Solubility of no2 >Solubility of no2
Solubility Of No2. 124 102 molLmin d. LC50 67 ppmNO24H. No information found Reproductive Effects. 2 C2H2g 5 O2g 4 CO2g 2 H2Og 1 mole C2H2 2604 g C2H2.
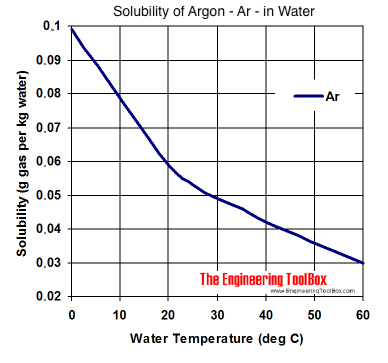 Solubility Of Gases In Water From engineeringtoolbox.com
Solubility Of Gases In Water From engineeringtoolbox.com
16 104 molLmin e. N2H4l 3 O2g 2 NO2g 2 H2Og 1 mole N2H4 3205 N2H4. 4292015 HW16 ClaireMelvin CH101section003Spring2015 InstructorJacobCW. Below the reaction in terms of nitrite ions NO2 with tetrachloroplatinate complex ion II. Every solution has a solubility product constant Ksp to represent its state in dynamic equilibrium. 2ΔG f NO2 g - 1ΔG f N2O4 g 2513 - 19782 478 kJ 478 kJ nonspontaneous From ΔG ΔH - TΔS.
The problem requires that you determine the mass of oxygen O2 needed for the reaction to occur.
Assume 475 grams of acetylene C2H2 reacts with oxygen O2 to produce carbon dioxide CO2 and water H2O. Nitrogen dioxide is a chemical compound with the formula NO 2It is one of several nitrogen oxides. Solubility Equation Determine the state of each substance gasg liquidl solidnon-solubles aqueoussolubleaq by using the solubility rules or a solubility table. NO 2 is an intermediate in the industrial synthesis of nitric acid millions of tons of which are produced each year for use primarily in the production of fertilizersAt higher temperatures it is a reddish-brown gas. Not listed by ACGIH IARC NTP or CA Prop 65. This occurs because the lower solubility of oxygen with increasing temperatures in a freely vented system decreases the corrosion rate faster than the rise in temperature increases it.
 Source: semanticscholar.org
Source: semanticscholar.org
NO 2 is an intermediate in the industrial synthesis of nitric acid millions of tons of which are produced each year for use primarily in the production of fertilizersAt higher temperatures it is a reddish-brown gas. Using GenScripts proprietary FlexPeptide technology we are able to synthesize any peptide modification at competitive prices. Write it by breaking all the. No information found Teratogenicity. The half-life of this reaction is approximately a.
 Source: europeanmedical.info
Source: europeanmedical.info
Based on the N-O bond distances of 1199 1211 and 1406 pm I would estimate the actual bond. Back to list of. Incompatibilities Reactivities Combustible material water chlorinated hydrocarbons carbon. Oxidation number change method ion-electron method also called the half-reaction method. See solubility in water diethyl ether a cetone.

The differences here include the number of oxygens attached to nitrogen. 124 102 molLmin d. There are two common techniques for balancing redox equations. Trans effect strength of several ligands can be sorted as follows. Chemical Principles 6th ed.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
However in many closed systems the dissolved oxygen entering the system in the makeup water cannot be freely vented resulting in the release of oxygen at points of high heat transfer which may cause severe. Not listed by ACGIH IARC NTP or CA Prop 65. 16 104 molLmin e. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. HNO3 H2O -.
 Source: researchgate.net
Source: researchgate.net
Oxidation number change method ion-electron method also called the half-reaction method. View Homework Help - HW16 Solubility Electrolytes and Net Ionic Equations from CH 101 at North Carolina State University. No information found Reproductive Effects. HNO3 H2O -. Calculate the number of kJ required for the reaction shown below if you begin with 48 grams of oxygen O2 and the ΔHrxn 180 kJ.
 Source: semanticscholar.org
Source: semanticscholar.org
Soluble in benzene and chloroform. 32 104 molLmin c. It has a role as an antimicrobial food preservative an antihypertensive agent a food antioxidant a poison and an antidote to cyanide poisoning. LC50 67 ppmNO24H. Back to list of.
 Source: semanticscholar.org
Source: semanticscholar.org
16 104 molLmin e. Noncombustible LiquidGas but will accelerate the burning of combustible materials. Back to list of. Solubility of Polycyclic Aromatic Hydrocarbons in Pure and Organic Solvent Mixtures-Revised and Updated. This occurs because the lower solubility of oxygen with increasing temperatures in a freely vented system decreases the corrosion rate faster than the rise in temperature increases it.
 Source: researchgate.net
Source: researchgate.net
Learn about the solubility equilibrium and how to use a Ksp in calculations determining Ksp. None of these ANSWER. The differences here include the number of oxygens attached to nitrogen. Every solution has a solubility product constant Ksp to represent its state in dynamic equilibrium. Nitric oxide nitrogen oxide or nitrogen monoxide is a colorless gas with the formula NOIt is one of the principal oxides of nitrogenNitric oxide is a free radical.
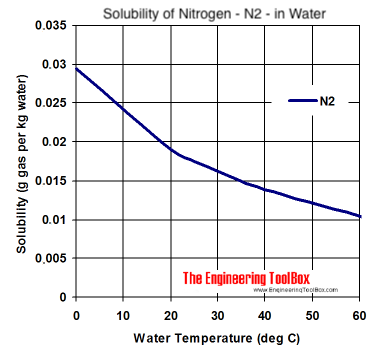 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Chemical Principles 6th ed. Trans effect strength of several ligands can be sorted as follows. Assume 475 grams of acetylene C2H2 reacts with oxygen O2 to produce carbon dioxide CO2 and water H2O. Identify the following reaction as an endothermic reaction exothermic reaction or both endothermic and exothermic. Soluble in benzene and chloroform.
 Source: researchgate.net
Source: researchgate.net
Not listed by ACGIH IARC NTP or CA Prop 65. Assume 171 grams of diborane B2H6 reacts with. It is a nitrite salt and an inorganic sodium salt. Nitric oxide nitrogen oxide or nitrogen monoxide is a colorless gas with the formula NOIt is one of the principal oxides of nitrogenNitric oxide is a free radical. Stability and Reactivity Data Stability.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title solubility of no2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.