Solid copper sulfate molar mass
Home » chemical » Solid copper sulfate molar mass >Solid copper sulfate molar mass
Solid Copper Sulfate Molar Mass. Atomic number the number of protons in the nucleus of an atom. 15960 gmol anhydrous 249685 gmol pentahydrate. Implementing POGIL in the Lecture and the Science. What is the molarity of a salt solution made by dissolving 2800 mg of NaCl in 200 mL of water.
 What Is The Molar Mass Of Copper Ll Sulfate Socratic From socratic.org
What Is The Molar Mass Of Copper Ll Sulfate Socratic From socratic.org
Calculate the moles of copper sulfate in 25000 mL of 0020 mol L-1 copper sulfate solution. Copper hydroxide is also called cupric hydroxide. Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Anhydrous copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass. 3386 gcm 3 anhydrous 251 gcm 3 dihydrate Melting point. Both hydrated and anhydrous copper.
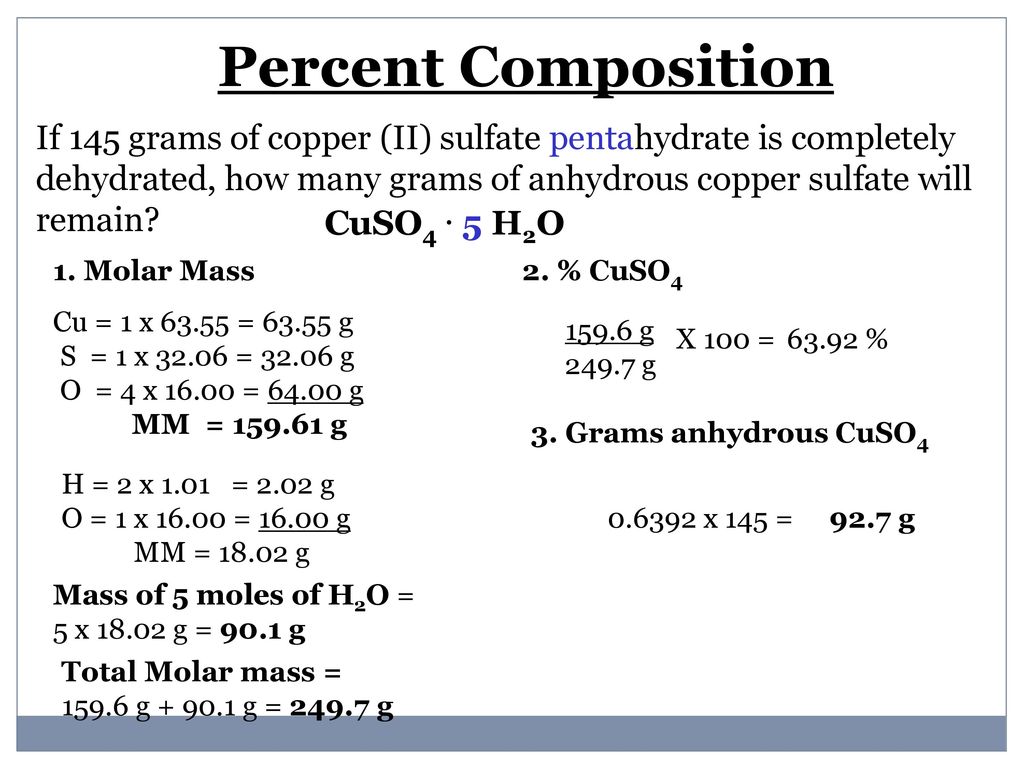
Anhydrous copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass.
When solid barium chloranilate is added to a solution containing sulfate barium sulfate is. Atomic mass the mass of an atom expressed in atomic mass units amu. Schroeder JD and Greenbowe TJ. This dark blue to purple solid is a salt of the metal complex CuNH 3 4 H 2 O 2It is closely related to Schweizers reagent which is used for the production of cellulose fibers in the production of rayon. Molar mass xH2O 3221 23x2 321 16x4 180 X 18018 10. If a reversible reaction is exothermic in one direction it is endothermic in the opposite direction.
 Source: youtube.com
Source: youtube.com
Basically adsorption is a mass transfer process by which a substance is transferred from the liquid phase to the surface of a solid and becomes bound by physical andor chemical interactions Kurniawan and Babel 2003. 771 K anhydrous 100 C dehydration of dihydrate Boiling point. If 602 grams of Hg combines completely with 240 grams of Br to form a compound what is the percent composition of Hg in the compound. Schroeder JD and Greenbowe TJ. Anhydrous CuSO 4 has a grey-white powdery appearance whereas the pentahydrate has a bright blue colour.
 Source: sciencing.com
Source: sciencing.com
Copper hydroxide reacts with sulfuric acid to form copper sulfate and water. The mass of water lost during heating can be determined and the. Both hydrated and anhydrous copper. Large crystals 1040 mm small crystals 210 mm snow crystals. Method 9035 is applicable to ground water drinking and surface waters and domestic and industrial wastes containing 10 to 400 mg sulfatel.
 Source: youtube.com
Source: youtube.com
What is the molarity of a solution prepared by dissolving 1416 g of citric acid. By adding solvent to a measured portion of a more concentrated stock solution we can achieve a particular concentration. Extract the data from the question. O and magnesium sulfate MgSO. If a reversible reaction is exothermic in one direction it is endothermic in the opposite direction.
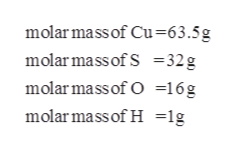 Source: bartleby.com
Source: bartleby.com
If a reversible reaction is exothermic in one direction it is endothermic in the opposite direction. Mark Ott Dilution is also a common means of preparing solutions of a desired concentration. What is the question asking you to calculate. The water molecules are loosely attached to the salt and can be removed upon heating yielding the anhydrous salt salt without water. When solid barium chloranilate is added to a solution containing sulfate barium sulfate is.
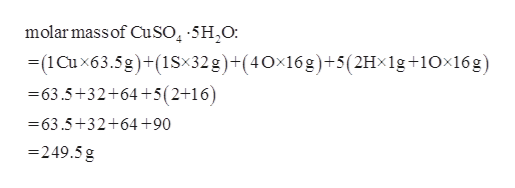 Source: bartleby.com
Source: bartleby.com
Large crystals 1040 mm small crystals 210 mm snow crystals. Atomic mass the mass of an atom expressed in atomic mass units amu. Atomic number the number of protons in the nucleus of an atom. TetraamminecopperII sulfate is the salt with the formula CuNH 3 4SO 4 H 2 O. The water molecules are loosely attached to the salt and can be removed upon heating yielding the anhydrous salt salt without water.
 Source: socratic.org
Source: socratic.org
Schroeder JD and Greenbowe TJ. This is just the ratio of the molar mass of CH 4 16 g to that of two moles of dioxygen 2 x 32 g Thus 64 g 16 g 41 40. How many grams of solid sodium hydroxide are required to make 17500 mL of a 05100 M NaOH solution. Impact of Learners Prior Knowledge on Their Use of Chemistry Computer Simulations. 1266 K anhydrous decomposes Solubility in water.
 Source: youtube.com
Source: youtube.com
The initial mass of copper used in the experiment was 2004 grams and. Calculate the moles of copper sulfate in 25000 mL of 0020 mol L-1 copper sulfate solution. Atomic mass unit amu a unit of mass equal to 112 the mass of the carbon isotope with mass number 12 approximately 16604 x 10E-24 gram. How many grams of solid sodium hydroxide are required to make 17500 mL of a 05100 M NaOH solution. Basically adsorption is a mass transfer process by which a substance is transferred from the liquid phase to the surface of a solid and becomes bound by physical andor chemical interactions Kurniawan and Babel 2003.
 Source: slideplayer.com
Source: slideplayer.com
If a reversible reaction is exothermic in one direction it is endothermic in the opposite direction. What is the molarity of a solution prepared by dissolving 1416 g of citric acid. What is the molarity of a salt solution made by dissolving 2800 mg of NaCl in 200 mL of water. Copper sulfate pentahydrate CuSO45H2O or CuH10O9S CID 24463 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. For example copperII pentahydrate CuSO_4 5H_2O is blue.
 Source: youtube.com
Source: youtube.com
The initial mass of copper used in the experiment was 2004 grams and. O will be studied. Both hydrated and anhydrous copper. What is the question asking you to calculate. Upper Saddle River NJ.
 Source: youtube.com
Source: youtube.com
Various low-cost adsorbents derived from agricultural waste industrial by-product natural material or modified biopolymers have been recently developed and applied for. O and magnesium sulfate MgSO. The densities of the anhydrous and pentahydrate forms are 36 grams per cubic centimetre and 2286 gcm-3. Calculate the mass ratio of CH 4 to O 2 required for complete combustion. 993 C 1819 F.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title solid copper sulfate molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.