Sodium nitrite hazards
Home » chemical » Sodium nitrite hazards >Sodium nitrite hazards
Sodium Nitrite Hazards. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Preservatives such as sodium nitrite in the product covered in Chapter 13. These substances have been attributed E numbers E250 E249 E251 and E252 respectively. Specify a facility by using any combination of facility name and geographic location.
 Sodium Nitrite 99 999 Trace Metals Basis 7632 00 0 From sigmaaldrich.com
Sodium Nitrite 99 999 Trace Metals Basis 7632 00 0 From sigmaaldrich.com
Sodium nitrite is used to speed up the curing of meat and also impart an attractive pink color. These are sodium and potassium nitrite and sodium and potassium nitrate. Regarding the health hazards that may result from exposure. Crews in Encyclopedia of Food Safety 2014. 2003 CERCLA Priority List of Hazardous Substances. The Comprehensive Environmental Response Compensation and Liability Act CERCLA section 104 i as amended by the Superfund Amendments and Reauthorization Act SARA requires ATSDR and the EPA to prepare a list in order of priority of substances that are most commonly found at facilities on the National Priorities List NPL and which.
990 sodium nitrite 06 sodium nitrate 01 sodium chloride and sodium sulfate and 01 water.
It is on the World Health Organizations List of Essential Medicines. Burns violently with explosions that may spatter the material. The particle size selective characteristics are determined by the type of cyclone used together with the sampling flow rate. Adult Until sodium nitrite becomes available break one ampule of amyl nitrite into a cloth. Hydrogen peroxide 275 to 52. They are formed by the reaction of nitrite with secondary amines.
 Source: www2.atmos.umd.edu
Source: www2.atmos.umd.edu
A new ampule of amyl nitrite should be broken into a cloth every 3 minutes. 2003 CERCLA Priority List of Hazardous Substances. Nitrite reacts with the meat myoglobin to cause color changes first converting to. Sodium nitrite is used to speed up the curing of meat and also impart an attractive pink color. Out of every minute hold the cloth containing amyl nitrite in front of the patients mouth for 30 seconds and then remove it for 30 seconds until sodium nitrite can be administered.

The particle size selective characteristics are determined by the type of cyclone used together with the sampling flow rate. A Dorr-Oliver cyclone set to a flow rate of 17 Lmin can be used in order to meet the specifications described in Table Z-3 Mineral Dusts of 29 CFR 19101000 footnote e Footnote e states that both concentration and percent quartz for the application of the. Peroxomonosulfuric acid nitrobenzene sodium nitrite aluminum chloride peroxydisulfuric acid 13 - butadiene boron trifluoride diethyl ether. It should be noted that nitrates may also form N-nitrosamines through reduction to nitrites by saliva or enzymes. These substances have been attributed E numbers E250 E249 E251 and E252 respectively.
Crews in Encyclopedia of Food Safety 2014. Peroxomonosulfuric acid nitrobenzene sodium nitrite aluminum chloride peroxydisulfuric acid 13 - butadiene boron trifluoride diethyl ether. 108-95-2 LD50 Oral - Rat - 3170 mgkg BehavioralConvulsions or effect on seizure threshold. Sodium nitrite is an efficient drug in case of cyanide poisoningIt is used together with sodium thiosulfate. Nitrite reacts with the meat myoglobin to cause color changes first converting to.
 Source: brecklandscientific.co.uk
Source: brecklandscientific.co.uk
Peroxomonosulfuric acid nitrobenzene sodium nitrite aluminum chloride peroxydisulfuric acid 13 - butadiene boron trifluoride diethyl ether. A new ampule of amyl nitrite should be broken into a cloth every 3 minutes. Furthermore other potential chemical hazards may include substances that are safe or used in processing at certain levels but can cause illness or injury if consumed at too high of a concentration such as sodium nitrite or antimicrobial solutions used in intervention steps. LaMotte NitriteNitrogen Reagent 1 LaMotte NitriteNitrogen Reagent 2 LaMotte NitriteNitrogen Reagent 3 LaMottehosphorusP Reagent 2 LaMotte Phosphorus Reagent 3 Tablets LaMotte Potassium Reagent B Tablets LaMotte Potassium Reagent C LaMotte Soil Flocculating Reagent LaMotte Sulfate Test Solution LaMotte Universal. These substances have been attributed E numbers E250 E249 E251 and E252 respectively.
 Source: chemworld.com
Source: chemworld.com
Out of every minute hold the cloth containing amyl nitrite in front of the patients mouth for 30 seconds and then remove it for 30 seconds until sodium nitrite can be administered. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Hazardous decomposition productsCarbon oxides. Regarding the health hazards that may result from exposure. Sodium nitrate is the chemical compound with the formula Na N O 3This alkali metal nitrate salt is also known as Chile saltpeter large deposits of which were historically mined in Chile to distinguish it from ordinary saltpeter potassium nitrateThe mineral form is also known as nitratine nitratite or soda niter.

The particle size selective characteristics are determined by the type of cyclone used together with the sampling flow rate. Used for making gasoline additives electric power cable sodium lamps other chemicals. Hach Sodium Hydroxide Solution 0075 N. Sodium perborate and its monohydrate sodium persulfate. Crews in Encyclopedia of Food Safety 2014.

We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Sodium amide NaNH 2 can undergo oxidation on exposure to air to give sodium nitrite in a mixture that is unstable and may explode. LaMotte NitriteNitrogen Reagent 1 LaMotte NitriteNitrogen Reagent 2 LaMotte NitriteNitrogen Reagent 3 LaMottehosphorusP Reagent 2 LaMotte Phosphorus Reagent 3 Tablets LaMotte Potassium Reagent B Tablets LaMotte Potassium Reagent C LaMotte Soil Flocculating Reagent LaMotte Sulfate Test Solution LaMotte Universal. It is on the World Health Organizations List of Essential Medicines. These are sodium and potassium nitrite and sodium and potassium nitrate.
 Source: draxe.com
Source: draxe.com
This is our newest publication and has been created to support the school technician profession in Scotland. Sodium nitrate is a white deliquescent solid very soluble in water. Used for making gasoline additives electric power cable sodium lamps other chemicals. A Dorr-Oliver cyclone set to a flow rate of 17 Lmin can be used in order to meet the specifications described in Table Z-3 Mineral Dusts of 29 CFR 19101000 footnote e Footnote e states that both concentration and percent quartz for the application of the. Sulfuric acid H 2 SO 4 should be.

Crews in Encyclopedia of Food Safety 2014. Preservatives such as sodium nitrite in the product covered in Chapter 13. 2003 CERCLA Priority List of Hazardous Substances. Sodium nitrate is the chemical compound with the formula Na N O 3This alkali metal nitrate salt is also known as Chile saltpeter large deposits of which were historically mined in Chile to distinguish it from ordinary saltpeter potassium nitrateThe mineral form is also known as nitratine nitratite or soda niter. Health Hazard Information Acute Health Effects The following acute short-term health effects may occur immediately or shortly after exposure to Sodium Nitrite.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
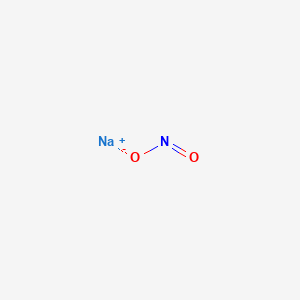
Sodium nitrite. Furthermore other potential chemical hazards may include substances that are safe or used in processing at certain levels but can cause illness or injury if consumed at too high of a concentration such as sodium nitrite or antimicrobial solutions used in intervention steps. 990 sodium nitrite 06 sodium nitrate 01 sodium chloride and sodium sulfate and 01 water. Peroxomonosulfuric acid nitrobenzene sodium nitrite aluminum chloride peroxydisulfuric acid 13 - butadiene boron trifluoride diethyl ether. Examples of NFPA Class 2 oxidizers include.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium nitrite hazards by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.