Sodium chromate solubility
Home » chemical » Sodium chromate solubility >Sodium chromate solubility
Sodium Chromate Solubility. G H 2 O 150. Write a balanced equation for the following. Reference Tables for Physical SettingChemistry 2011 Edition 4 Table H Vapor Pressure of Four Liquids 0 25 50. Sodium chromate was also available as a chemically pure grade as the tetrahydrate and in solutions.
 Sodium Chromate Wikipedia From en.wikipedia.org
Sodium Chromate Wikipedia From en.wikipedia.org
Calcium chromate solubility is 170 gL. In low pH silver chromate solubility grows due to the protonation of chromate anions in. Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL. Solubility of strontium and strontium compounds. Before titration small amount of sodium or potassium chromate is added to the solution making its slightly yellow in color. Aqueous barium chloride reacts with aqueous sodium chromate to form aqueous sodium chloride plus solid barium chromate.
Solubility and pH appear to be the primary determinants of the capacity of individual chromium compounds to elicit an.
The table below provides information on the variation of solubility of different substances mostly inorganic compounds in water with temperature at one atmosphere pressureUnits of solubility are given in grams per 100 millilitres of water g100 mL unless shown otherwise. Then for a saturated solution we have Ag 2S CrO_42 S Substituting this into Eq 5b above. What is the maximum concentration of chromate ion that is allowed before silver chromate will precipitate if the silver ion concentration is 78 x 10-4 M. Read MSDS for details. Contains crystalline silica Use proper engineering controls work practices and personal protective equipment to prevent exposure to wet or dry product. The LD 50 of rats for calcium arsenite is 20 mg kg body weight.
 Source: slideplayer.com
Source: slideplayer.com
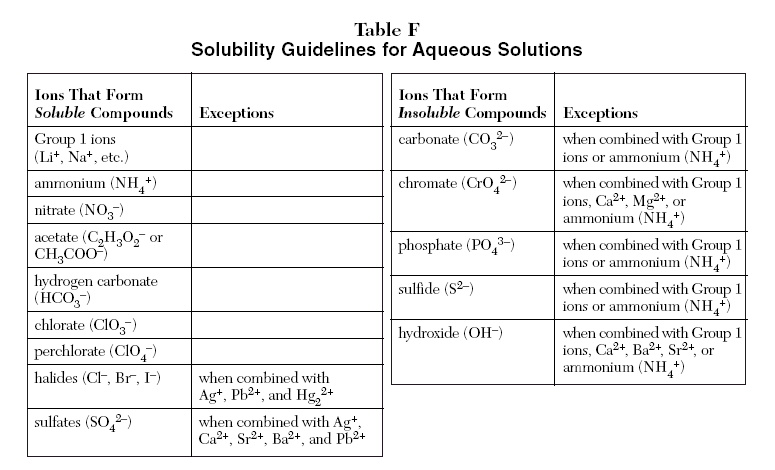
What is the solubility of silver chromate in a solution that is 024 M in silver nitrate. Chromate CrO 4 2 when combined. Of Fe3 Green Pre. The most significant strontium mineral is celestite strontium sulphate. Practically all sodium potassium.
 Source: kentchemistry.com
Source: kentchemistry.com
How many leadII and iodate ions are present in 38 L of a saturated solution of leadII iodate. Strontium is water insoluble but it does react with water. Strontium compounds can be water soluble. The table below provides information on the variation of solubility of different substances mostly inorganic compounds in water with temperature at one atmosphere pressureUnits of solubility are given in grams per 100 millilitres of water g100 mL unless shown otherwise. Why is strontium present in water.
 Source: researchgate.net
Source: researchgate.net
Contains crystalline silica Use proper engineering controls work practices and personal protective equipment to prevent exposure to wet or dry product. Then for a saturated solution we have Ag 2S CrO_42 S Substituting this into Eq 5b above. Those that absorb so much water that they will dissolve in it and form a concentrated solution are called deliquescent eg sodium hydroxide trichloroacetic acid. G H 2 O 150. Most often the solvent is a liquid which can be a pure substance or a mixture.
 Source: sciencemadness.org
Source: sciencemadness.org
Its solubility in water rises more than tenfold between 0 C and 32384 C where it reaches a maximum of 497 g100 mL. Examples include strontium carbonate with a water solubility of 10 mgL and strontium chromate with a water solubility of 9 mgL. Reference Tables for Physical SettingChemistry 2011 Edition 4 Table H Vapor Pressure of Four Liquids 0 25 50. Practically all sodium potassium. The solubility of solid chromate Ag_2 CrO_4 which has K_sp 90 times 10 -12 text at 25circ C is determined in water and in two different aqueous solutions.
 Source: en.wikipedia.org
Source: en.wikipedia.org
0 KI NaNO 3 KNO 3 NH 4 Cl HCl KCl NaCl KClO 3 NH 3 SO 2 Table G Solubility Curves at Standard Pressure. During titration as long as chlorides are present concentration of Ag is too low for silver chromate formation. Write a balanced equation for the following. Near equivalence point concentration of silver cations rapidly. Sodium and chlorine content is low.
 Source: youtube.com
Source: youtube.com
Those that absorb so much water that they will dissolve in it and form a concentrated solution are called deliquescent eg sodium hydroxide trichloroacetic acid. How many leadII and iodate ions are present in 38 L of a saturated solution of leadII iodate. 1 Noted the colour Colourless Absenceof Cu2 Fe2 Fe3 Mn2 Co2 Ni2 Blue Pre. Then for a saturated solution we have Ag 2S CrO_42 S Substituting this into Eq 5b above. All nitrates are soluble.
 Source: youtube.com
Source: youtube.com
All nitrates are soluble. SALT ANALYSIS SCHEME OF ANALYSIS EXPERIMENT OBSERVATION INFERENCE PRELIMINARY EXAMINATIONS. Sodium chromate was also available as a chemically pure grade as the tetrahydrate and in solutions. Solubility and pH appear to be the primary determinants of the capacity of individual chromium compounds to elicit an. Aqueous barium chloride reacts with aqueous sodium chromate to form aqueous sodium chloride plus solid barium chromate.
 Source: sciencedirect.com
Source: sciencedirect.com
The substances are listed in alphabetical order. Reference Tables for Physical SettingChemistry 2011 Edition 4 Table H Vapor Pressure of Four Liquids 0 25 50. Various calcium compounds may be toxic. Strontium compounds can be water soluble. Then for a saturated solution we have Ag 2S CrO_42 S Substituting this into Eq 5b above.
If the compound is moderately soluble some of this amount will dissolve. Solubility and pH appear to be the primary determinants of the capacity of individual chromium compounds to elicit an. Place one small spatula of the compound in 1 mL of water. Sodium sulfate has unusual solubility characteristics in water. Pb 2 aq SO 4 2 aq PbSO 4 s white PbSO 4 s.
 Source: sciencedirect.com
Source: sciencedirect.com
Pb 2 aq SO 4 2 aq PbSO 4 s white PbSO 4 s. The precipitate is partially soluble in concentrated sulfuric acid and dilute nitric acid and highly soluble in hot sodium hydroxide or acetate buffer. Why is strontium present in water. The most significant strontium mineral is celestite strontium sulphate. At this point the solubility curve changes slope and the solubility becomes almost independent of temperature.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium chromate solubility by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.