Sodium chloride hazards
Home » chemical » Sodium chloride hazards >Sodium chloride hazards
Sodium Chloride Hazards. ANHYDROUS HYDROGEN CHLORIDE is an anhydrous no water strong acid. In the following demonstrations a 25 liter bottle is filled with chlorine gas. To learn more about the Structure of Sodium Sulfite Molecules along with the Preparation Properties Health hazards Uses and FAQs Visit BYJUS for complete information. The human body requires a small amount of sodium to conduct nerve impulses contract and relax muscles and maintain the proper balance of water and minerals.
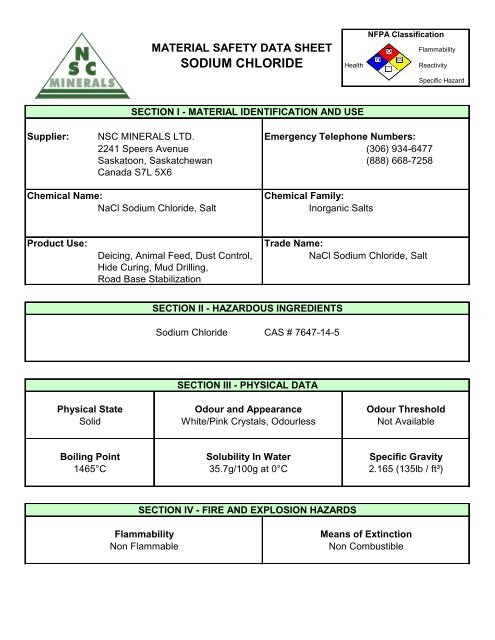
 Sodium Chloride 7 Msds From studylib.net
Sodium Chloride 7 Msds From studylib.net
Developmental and reproductive toxicity. Visit BYJUS for more information. It is also a food preservative as bacteria cant thrive in the presence of a high amount of salt. Ingredients linked to developmental and reproductive toxicity a broad class of health effects that range from. The same principle was used in another 2050 patent to produce concentrated slurries of the pentahydrate NaClO5 H 2 O. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound.
It is also a food preservative as bacteria cant thrive in the presence of a high amount of salt.
The crystals are vacuum-dried to produce a free-flowing crystalline powder. Developmental Reproductive Toxicity. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. Class D fires only involving combustible metals - magnesium sodium spills and in depth potassium sodium-potassium alloys uranium and powdered aluminum. Holding the extinguisher with the nozzle pointing away from you pull out the pin located below the. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
 Source: studylib.net
Source: studylib.net
With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. When depleted in the body sodium must be replaced in order to maintain intracellular osmolarity nerve conduction muscle contraction and normal renal function. In the following demonstrations a 25 liter bottle is filled with chlorine gas. Visit BYJUS for more information. Sodium Chloride is an inorganic salt also called table salt.
 Source: safety365.sevron.co.uk
Source: safety365.sevron.co.uk
Developmental and reproductive toxicity. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. When depleted in the body sodium must be replaced in order to maintain intracellular osmolarity nerve conduction muscle contraction and normal renal function. Sodium Acetate Anhydrous is the anhydrous sodium salt form of acetic acidSodium acetate anhydrous disassociates in water to form sodium ions Na and acetate ions. Hazards of materials in their facility.

It is a white crystalline solid with a sulfurous Salty taste. The solution is vacuum evaporated at 4050 C and 12 mmHg until the dihydrate crystallizes out. Typically a 35 solution by. It is also a food preservative as bacteria cant thrive in the presence of a high amount of salt. Reacts with sulfides carbides borides and phosphides to generate toxic.
 Source: chemedx.org
Source: chemedx.org
Holding the extinguisher with the nozzle pointing away from you pull out the pin located below the. The solution is vacuum evaporated at 4050 C and 12 mmHg until the dihydrate crystallizes out. Developmental and reproductive toxicity. Hazards of materials in their facility. Some sodium chloride precipitates and is removed by fitration.
 Source: safety365.sevron.co.uk
Source: safety365.sevron.co.uk
To learn more about the Structure of Sodium Sulfite Molecules along with the Preparation Properties Health hazards Uses and FAQs Visit BYJUS for complete information. Reacts with sulfides carbides borides and phosphides to generate toxic. When a sodium atom transfers an electron to a chlorine atom forming a sodium cation Na and a chloride anion Cl- both ions have complete valence shells and are energetically more stable. When depleted in the body sodium must be replaced in order to maintain intracellular osmolarity nerve conduction muscle contraction and normal renal function. To learn more about the Structure of Sodium Sulfite Molecules along with the Preparation Properties Health hazards Uses and FAQs Visit BYJUS for complete information.
 Source: yumpu.com
Source: yumpu.com
Visit BYJUS for more information. The system is characterized by the diamond that is actually a square onpoint shape. Ingredients linked to cancer in government industry or academic studies or assessments. Sodium is the principal cation of the extracellular fluid and plays a large part in fluid and electrolyte replacement therapies. Some sodium chloride precipitates and is removed by fitration.

With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. Visit BYJUS for more information. Sodium Sulfite Na2SO3-Sodium Sulfite is an Ionic Salt with the Chemical Formula Na2SO3. When depleted in the body sodium must be replaced in order to maintain intracellular osmolarity nerve conduction muscle contraction and normal renal function. When a sodium atom transfers an electron to a chlorine atom forming a sodium cation Na and a chloride anion Cl- both ions have complete valence shells and are energetically more stable.
 Source: studylib.net
Source: studylib.net
When depleted in the body sodium must be replaced in order to maintain intracellular osmolarity nerve conduction muscle contraction and normal renal function. How is the rating displayed. Sodium Chloride is an inorganic salt also called table salt. Sodium Sulfite Na2SO3-Sodium Sulfite is an Ionic Salt with the Chemical Formula Na2SO3. Developmental Reproductive Toxicity.
 Source: yumpu.com
Source: yumpu.com
How is the rating displayed. Sodium chloride is the salt most responsible. Some sodium chloride precipitates and is removed by fitration. The molecular weight of sodium hydroxide is 40 gmol. It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps.

The solution is vacuum evaporated at 4050 C and 12 mmHg until the dihydrate crystallizes out. The solution is vacuum evaporated at 4050 C and 12 mmHg until the dihydrate crystallizes out. The crystals are vacuum-dried to produce a free-flowing crystalline powder. Some sodium chloride precipitates and is removed by fitration. It flavors food and is used as a binder and stabilizer.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium chloride hazards by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.