Sodium chlorate molar mass
Home » chemical » Sodium chlorate molar mass >Sodium chlorate molar mass
Sodium Chlorate Molar Mass. Percent composition can be calculated the chemical formula of a compound or it can be determined experimentally. In other applications it is mostly obsolete and has been. Do not attempt this reaction unless are a professionally qualified chemist and you have carried out a legally satisfactory hazard assessment. 2KClO 3 400C 2KCl 3O 2.
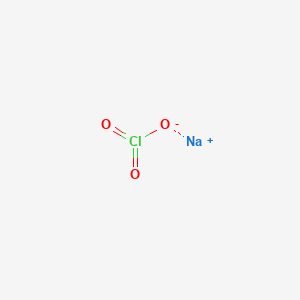
 How To Write The Formula For Sodium Chlorate Naclo3 Youtube From youtube.com
How To Write The Formula For Sodium Chlorate Naclo3 Youtube From youtube.com
This sample is from The Elements Collection an attractive and. This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Potassium chlorate is a compound containing potassium chlorine and oxygen with the molecular formula KClO 3In its pure form it is a white crystalline substance. For example lets find the molar mass of water H 2 O. Molar mass of al2s3.
By the way if its not actually the holidays and I havent updated this recently sorry about that.
Sodium chloride MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. Molar Mass of Frequently Calculated Chemicals. Potassium chlorate is a compound containing potassium chlorine and oxygen with the molecular formula KClO 3In its pure form it is a white crystalline substance. Melting point - the temperature at which a solid turns into a liquid. Sodium sulfate Na 2 SO 4. The molar mass of H_2O is 18 gmol The hydrogens make up 2g since each mole of hydrogen is 1g The oxygen makes up 16g.
 Source: coolgyan.org
Source: coolgyan.org
2KClO 3 400C 2KCl 3O 2. In a total of 14202 parts by mass of sodium sulfate 4598 parts are sodium 3206 parts are sulfur and 6400 parts are oxygen. In terms of atomic masses. This sample is from The Elements Collection an attractive and. Melting point - the temperature at which a solid turns into a liquid.
Boiling point - the temperature at which a liquid turns into a gas. For example lets find the molar mass of water H 2 O. Sodium silicate Na 2SiO 3 for. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. In other applications it is mostly obsolete and has been.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A salt ester or acylal of acetic acid is called acetate. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. However the decomposition of potassium chlorate is one route to O 2 and decomposition of potassium permanganate is another. The atomic mass of hydrogen is 100794 and. 55845 2 Standard state.
 Source: en.wikipedia.org
Source: en.wikipedia.org
2KMnO 4 214C K 2 MnO 4 MnO 2 O 2. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. In terms of atomic masses. If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate the mass explodes Mellor 2311. In other applications it is mostly obsolete and has been.

A dilute approximately 5 percent by volume solution of acetic acid produced by fermentation and oxidation of natural carbohydrates is called vinegar. Here is a video which discusses how to calculate percent. A mixture of potassium chlorate and sodium amide explodes Mellor 8258. How do you know the Order of Elements in a Chemical Formula What is Chemical Formula. Sodium chloride MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents.
 Source: youtube.com
Source: youtube.com
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The atomic mass can be found on the periodic table. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. By the way if its not actually the holidays and I havent updated this recently sorry about that. 106404 View Pricing Availability.

See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. By the way if its not actually the holidays and I havent updated this recently sorry about that. Sodium Na 1 Potassium K 1 Rubidium Rb 1 Cesium Cs 1 Beryllium Be 2 Magnesium Mg 2 Calcium Ca 2 Strontium Sr 2 Barium Ba 2 Aluminum Al 3 Common Anions Element Name symbol Ion Name symbol Ion Charge Fluorine Fluoride 1- Chlorine Chloride 1- Bromine Bromide 1- Iodine Iodide 1- Oxygen Oxide 2- Sulfur Sulfide 2- Nitrogen Nitride 3- Common Polyatomic Ions Ion. In a total of 14202 parts by mass of sodium sulfate 4598 parts are sodium 3206 parts are sulfur and 6400 parts are oxygen. 106404 View Pricing Availability.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
However the decomposition of potassium chlorate is one route to O 2 and decomposition of potassium permanganate is another. Molar Mass of Frequently Calculated Chemicals. 106404 View Pricing Availability. It is a strong oxidizing agent and its most important application is in safety matches. Sodium sulfate Na 2 SO 4.
 Source: youtube.com
Source: youtube.com
The atomic mass of hydrogen is 100794 and. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. After sodium chlorate it is the second most common chlorate in industrial use. Here is a video which discusses how to calculate percent. Sodium sulfate Na 2 SO 4.
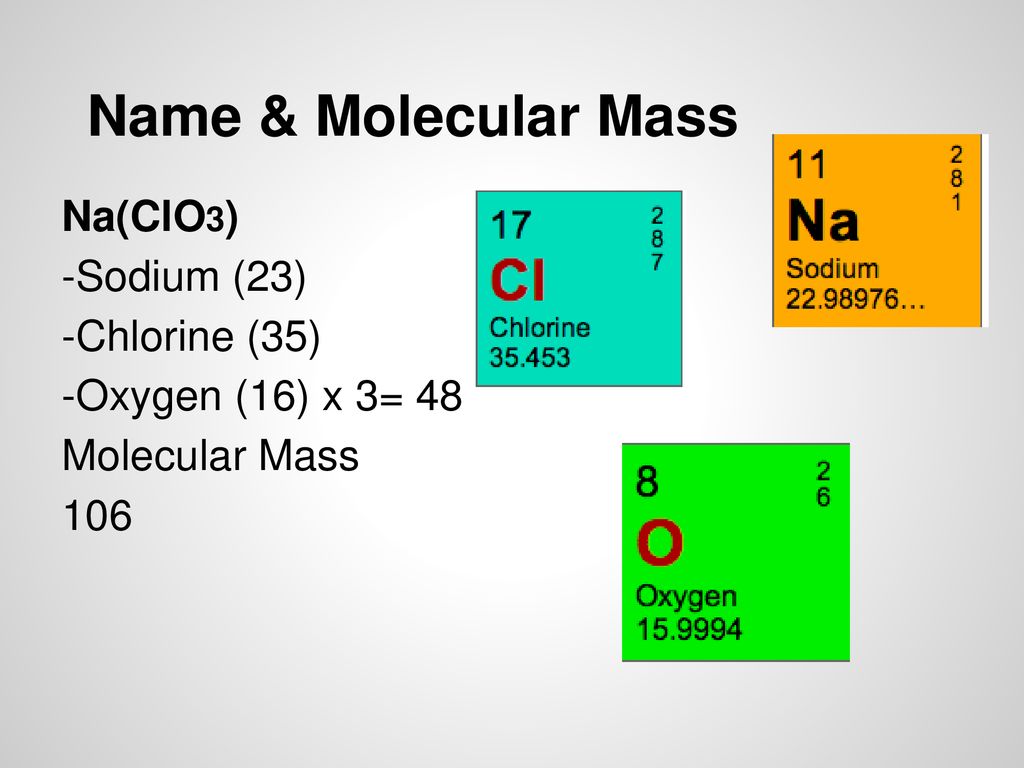 Source: slideplayer.com
Source: slideplayer.com
Sodium sulfate 2Na SO 4 2- Na 2 SO 4. B the chemical formula of the compound appears after the arrow. Since CH has a formula weight of 1302 divide molar mass by that. Boiling point - the temperature at which a liquid turns into a gas. It Hch3co2 name NaOH is Ionic.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium chlorate molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.