Sodium borohydride safety
Home » chemical » Sodium borohydride safety >Sodium borohydride safety
Sodium Borohydride Safety. It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited. Methane contains 4 equivalent C 3 axes and 3 equivalent C 2 axes. TiO 2 Rutile. Metal hydrides such as lithium aluminium hydride calcium hydride sodium borohydride and potassium borohydride are used extensively in the purification of chemicals.
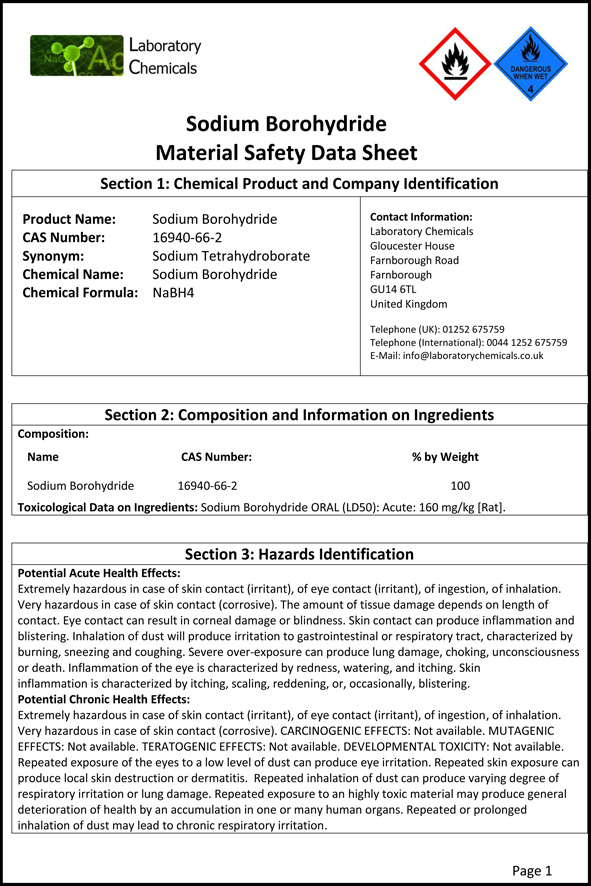 Sodium Borohydride Material Safety Data Sheet From sodiumborohydride.co.uk
Sodium Borohydride Material Safety Data Sheet From sodiumborohydride.co.uk
A safety data sheet or SDS is a standardized document that contains occupational safety and health data. CdCl 2 Cadmium chloride. Description SDS Pricing. There are 6 equivalent σ v planes. YCl 3 Yttrium trichloride. Boron products transformed into high value-added products by Eti Maden have a widespread use in conjunction with ongoing RD work.
Spontaneous ignition can result from solution of sodium borohydride in dimethylformamide.
Metal hydrides such as lithium aluminium hydride calcium hydride sodium borohydride and potassium borohydride are used extensively in the purification of chemicals. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. It can be used in solution in alcohols or even solution in water - provided the solution is alkaline. Because of the difficulty in regenerating the starting material relatively few derivatives of organic substances are used for purification. The primary aim of the cards is to promote the safe use of chemicals in the workplace. The free metal does not occur in nature and must be prepared from compounds.
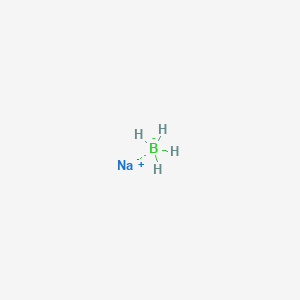
Depending on the type and amount of ligand present a coupling reaction using reductive amination can achieve immobilization yields of greater than 85. The ICSC project is a common undertaking between the World Health Organization WHO and. Its only stable isotope is 23 Na. In practice there is an additional step for safety reasons. The International Hazard Communication Standard HCS mandates that chemical manufacturers must communicate a chemicals hazard information to chemical handlers by providing a Safety Data Sheet.
 Source: studylib.net
Source: studylib.net
Hence methane belongs to the T d group. Click the Symmetry Operations above to view them in 3D. Using sodium tetrahydridoborate sodium borohydride Sodium tetrahydridoborate is a more gentle and therefore safer reagent than lithium tetrahydridoaluminate. Sodium is the sixth most abundant element in the Earths crust and exists. TiO 2 Rutile.
 Source: sodiumborohydride.co.uk
Source: sodiumborohydride.co.uk
The C 2 axes contain 3 equivalent S 4 axes. NaCl Sodium chloride. Hence methane belongs to the T d group. Bulk solutions of sodium borohydride are often prepared with excess sodium hydroxide which is corrosive. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.

Description SDS Pricing. Sodium is an alkali metal being in group 1 of the periodic table. NaCl Sodium chloride. Boron and boron products are used in construction and cement industry for strengthening and insulation. Bulk solutions of sodium borohydride are often prepared with excess sodium hydroxide which is corrosive.
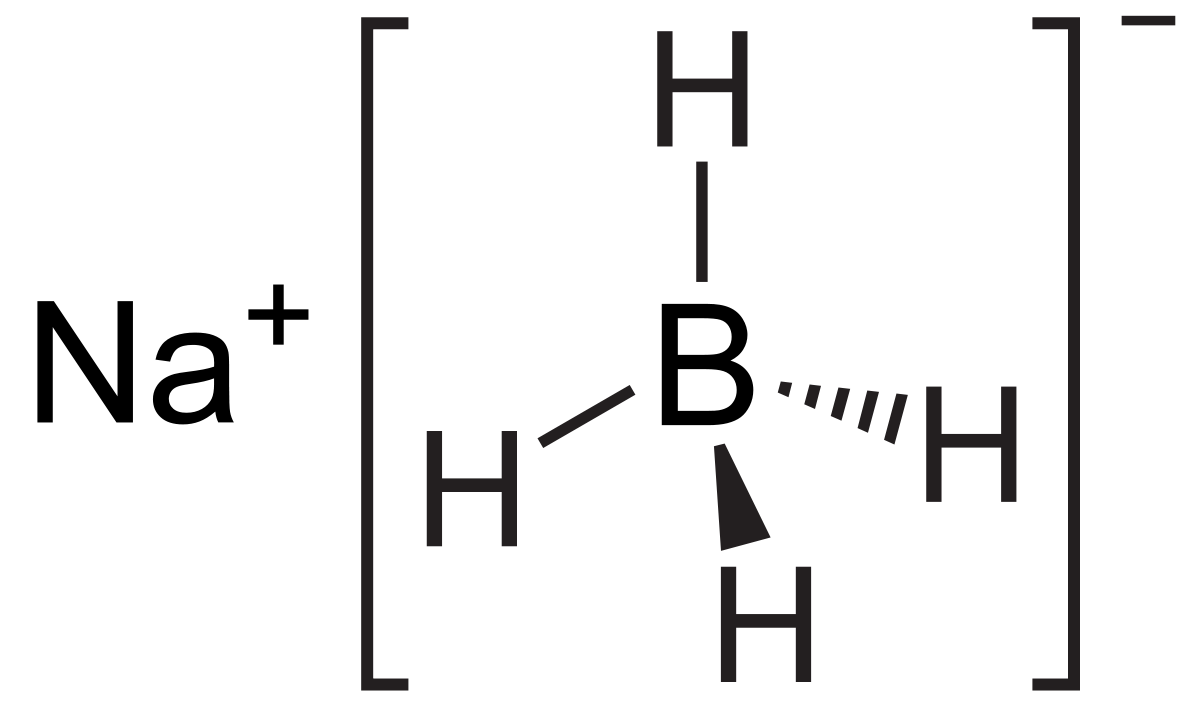 Source: en.wikipedia.org
Source: en.wikipedia.org
Company About Us Responsibility Events Press Releases Programs Careers Offices. Aluminium borohydride also known as aluminium tetrahydroborate is the chemical compound with the formula AlBH43. Boron products transformed into high value-added products by Eti Maden have a widespread use in conjunction with ongoing RD work. BiI 3 Bismuth triiodide. TiO 2 Rutile.
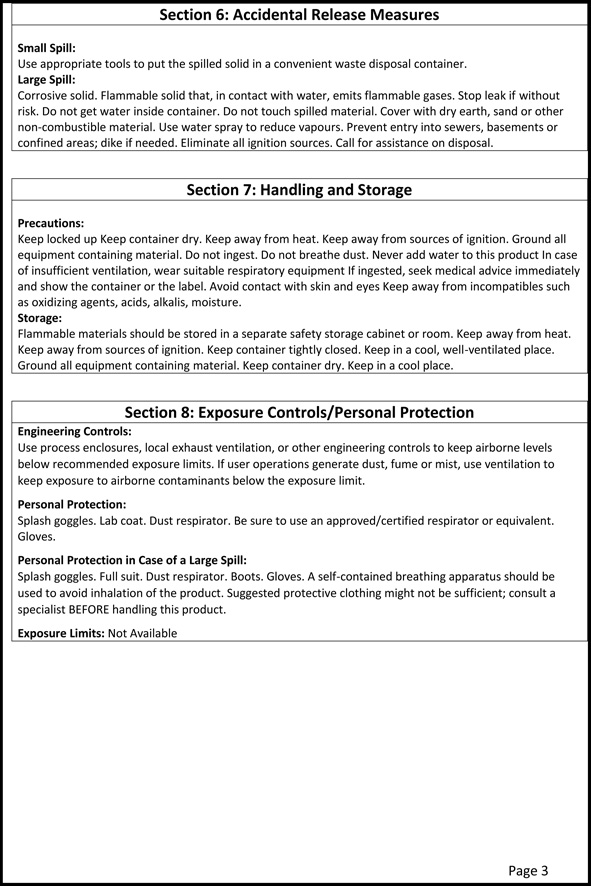 Source: sodiumborohydride.co.uk
Source: sodiumborohydride.co.uk
Boron and boron products are used in construction and cement industry for strengthening and insulation. The International Hazard Communication Standard HCS mandates that chemical manufacturers must communicate a chemicals hazard information to chemical handlers by providing a Safety Data Sheet. Empirical Formula Hill Notation. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
 Source: yumpu.com
Source: yumpu.com
Na 2 O Antifluorite. Sodium borohydride is a source of hydrogen or diborane which are both flammable. Depending on the type and amount of ligand present a coupling reaction using reductive amination can achieve immobilization yields of greater than 85. The primary aim of the cards is to promote the safe use of chemicals in the workplace. We are a leading supplier to the global Life Science industry with.
 Source: studylib.net
Source: studylib.net
Using sodium tetrahydridoborate sodium borohydride Sodium tetrahydridoborate is a more gentle and therefore safer reagent than lithium tetrahydridoaluminate. It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited. It can be used in solution in alcohols or even solution in water - provided the solution is alkaline. The C 2 axes contain 3 equivalent S 4 axes. Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms.
 Source: studylib.net
Source: studylib.net
Chemicals By Alphabet C We offer active chloride compound that are used in diverse industrial and laboratory applications. BiI 3 Bismuth triiodide. Aluminium borohydride also known as aluminium tetrahydroborate is the chemical compound with the formula AlBH43. I have a problem over describing the reaction conditions. It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited.
 Source: studylib.net
Source: studylib.net
Methane contains 4 equivalent C 3 axes and 3 equivalent C 2 axes. Acetyl coenzyme A sodium salt. Methane contains 4 equivalent C 3 axes and 3 equivalent C 2 axes. Depending on the type and amount of ligand present a coupling reaction using reductive amination can achieve immobilization yields of greater than 85. Aluminium borohydride also known as aluminium tetrahydroborate is the chemical compound with the formula AlBH43.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium borohydride safety by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.