Sodium borohydride hazards
Home » chemical » Sodium borohydride hazards >Sodium borohydride hazards
Sodium Borohydride Hazards. Abs 493 nm EC 9100 cm-1 M-1. The most common water sensitive chemicals include sodium potassium lithium metals and aluminum alkyls. Strader Eiko Hiraoka 2017 Immigration and Within-Group Wage Inequality. Sodium borohydride NaBH 4 sodium hydroxide NaOH l-ascorbic acid C 6 H 8 O 6 and potassium iodide KI were from Merck Germany.

QY increases 40-fold on binding to dsDNA. A standard reference material SRM 1515 Apple Leaves was obtained from the National Institute of Standards and Technology NIST USA. It has been tested as pretreatment for pulping of wood but is too costly to be commercialized. Sodium triacetoxyborohydride also known as sodium triacetoxyhydroborate commonly abbreviated STAB is a chemical compound with the formula NaCH 3 COO 3 BH. Like other borohydrides it is used as a reducing agent in organic synthesisThis colourless salt is prepared by protonolysis of sodium borohydride with acetic acid. When a plus sign is assigned to mixtures or solutions containing a material where the hazard to humans is significantly different from that of the pure material or where no hazard to humans is posed the material may be described using an alternative shipping name that represents the hazards posed by the material.
It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite.
NaBH 4 3 HO 2 CCH 3 NaBHO 2 CCH 3 3 3 H 2. This is the NFPA 704 for sodium borohydride. This periodical is devoted to the publication of fundamental research papers and publishes approximately 19000 pages of Articles Communications and Perspectives a year. It has been tested as pretreatment for pulping of wood but is too costly to be commercialized. Metal hydrides such as lithium aluminium hydride calcium hydride sodium borohydride and potassium borohydride are used extensively in the purification of chemicals. NaBH 4 3 HO 2 CCH 3 NaBHO 2 CCH 3 3 3 H 2.
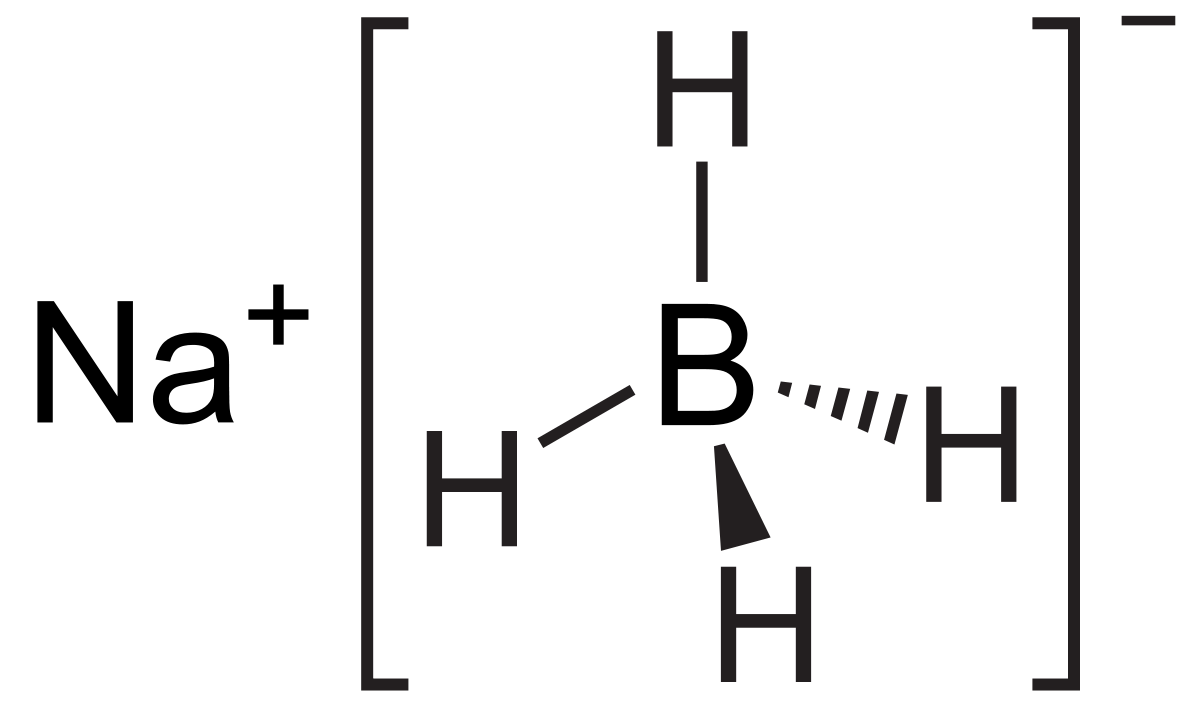 Source: en.wikipedia.org
Source: en.wikipedia.org
Chemistry Abbreviations Acronyms Basics Chemical Laws Molecules Periodic Table Projects Experiments Scientific Method Biochemistry Physical Chemistry Medical Chemistry Chemistry In Everyday Life Famous Chemists. A standard reference material SRM 1515 Apple Leaves was obtained from the National Institute of Standards and Technology NIST USA. This is the NFPA 704 for sodium borohydride. XSee Table 1 for the storage group codes descriptions and examples. Chemistry Abbreviations Acronyms Basics Chemical Laws Molecules Periodic Table Projects Experiments Scientific Method Biochemistry Physical Chemistry Medical Chemistry Chemistry In Everyday Life Famous Chemists.
 Source: studylib.net
Source: studylib.net
All glassware were soaked in 5. E1169 in H 2 O. XSee Table 1 for the storage group codes descriptions and examples. In order to effect decomposition the solid or aqueous solution is added to enough water to make the borohydride concentration less than 3 and then excess equivalents of dilute aqueous acetic acid are added drop wise with stirring under nitrogen. How Queuing Competition and Care Outsourcing Exacerbate and Erode Earnings Inequalities.

Sodium lithium and potassium metals sodium borohydride 26 Peroxide formers. E1374 spectral data are for the free. All glassware were soaked in 5. Sodium borohydride NaBH 4 sodium hydroxide NaOH l-ascorbic acid C 6 H 8 O 6 and potassium iodide KI were from Merck Germany. It has been tested as pretreatment for pulping of wood but is too costly to be commercialized.

This is the NFPA 704 for sodium borohydride. Metal hydrides such as lithium aluminium hydride calcium hydride sodium borohydride and potassium borohydride are used extensively in the purification of chemicals. Strader Eiko Hiraoka 2017 Immigration and Within-Group Wage Inequality. Many water reactive chemicals may also be corrosive toxic or pyrophoric. Abs 498 nm EC 10800 cm-1 M-1.
 Source: studylib.net
Source: studylib.net
Because of the difficulty in regenerating the starting material relatively few derivatives of organic substances are used for purification. Like other borohydrides it is used as a reducing agent in organic synthesisThis colourless salt is prepared by protonolysis of sodium borohydride with acetic acid. Both compounds are very weakly fluorescent in H 2 O. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. Tetraethyl-orthosilicate TEOS cetyl-trimethyl-ammonium bromide CTAB Hydrogen tetrachloroaurate III trihydrate HAuCl43H2O 99 was obtained from Acros.
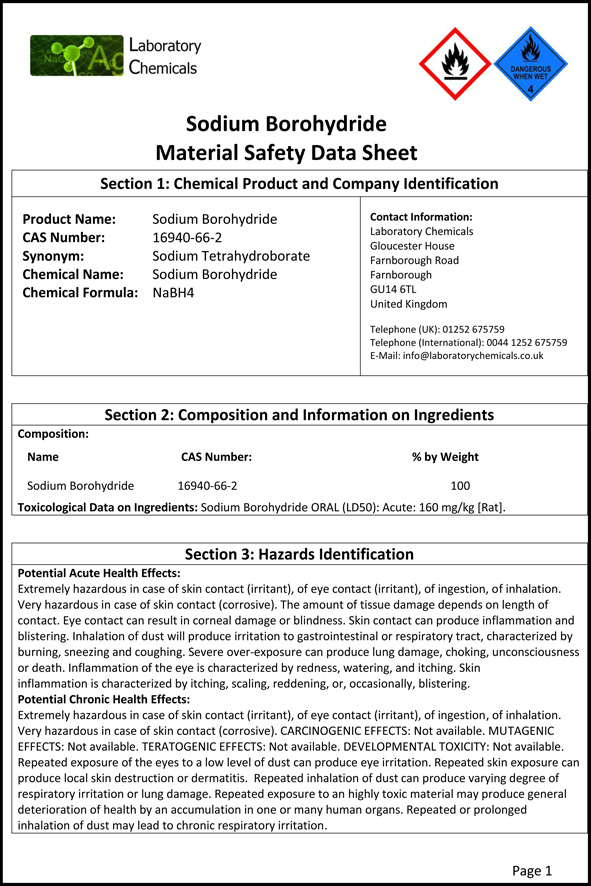 Source: sodiumborohydride.co.uk
Source: sodiumborohydride.co.uk
Make sure a Type D fire extinguisher is available. Abs 498 nm EC 10800 cm-1 M-1. Abs 493 nm EC 9100 cm-1 M-1. This is the NFPA 704 for sodium borohydride. Methyl alcohol 2-propyl alcohol ethyl alcohol Sodium borohydride resazurin sodium salt powder resazurin and tri-sodium citrate were supplied by Sigma-Aldrich St.
 Source: studylib.net
Source: studylib.net
How Queuing Competition and Care Outsourcing Exacerbate and Erode Earnings Inequalities. Methyl alcohol 2-propyl alcohol ethyl alcohol Sodium borohydride resazurin sodium salt powder resazurin and tri-sodium citrate were supplied by Sigma-Aldrich St. In order to effect decomposition the solid or aqueous solution is added to enough water to make the borohydride concentration less than 3 and then excess equivalents of dilute aqueous acetic acid are added drop wise with stirring under nitrogen. Sodium lithium and potassium metals sodium borohydride 26 Peroxide formers. Reduction of a commercially available pepsin-solubilized bovine dermal collagen Vitrogen 100 PureCols old product name with sodium 3Hborohydride provided radiolabeled collagen preparations with specific activities ranging from 71-120 muCimg collagen.
It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited. Sodium triacetoxyborohydride also known as sodium triacetoxyhydroborate commonly abbreviated STAB is a chemical compound with the formula NaCH 3 COO 3 BH. Strader Eiko Hiraoka 2017 Immigration and Within-Group Wage Inequality. When a plus sign is assigned to mixtures or solutions containing a material where the hazard to humans is significantly different from that of the pure material or where no hazard to humans is posed the material may be described using an alternative shipping name that represents the hazards posed by the material. Tetraethyl-orthosilicate TEOS cetyl-trimethyl-ammonium bromide CTAB Hydrogen tetrachloroaurate III trihydrate HAuCl43H2O 99 was obtained from Acros.
 Source: studylib.net
Source: studylib.net
QY increases 40-fold on binding to dsDNA. The four colored quadrants of the sign indicate the types of hazards presented by a material. Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. Metal hydrides and pyrophorics air or water reactive Examples. Methyl alcohol 2-propyl alcohol ethyl alcohol Sodium borohydride resazurin sodium salt powder resazurin and tri-sodium citrate were supplied by Sigma-Aldrich St.
 Source: sodiumborohydride.co.uk
Source: sodiumborohydride.co.uk
It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. Louis MO unless otherwise stated. The most common water sensitive chemicals include sodium potassium lithium metals and aluminum alkyls. XChemicals with multiple hazards are stored according to the primary hazard. Sodium borohydride calcium hydride lithium aluminum hydride.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium borohydride hazards by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.