Sodium bicarbonate to carbonic acid
Home » chemical » Sodium bicarbonate to carbonic acid >Sodium bicarbonate to carbonic acid
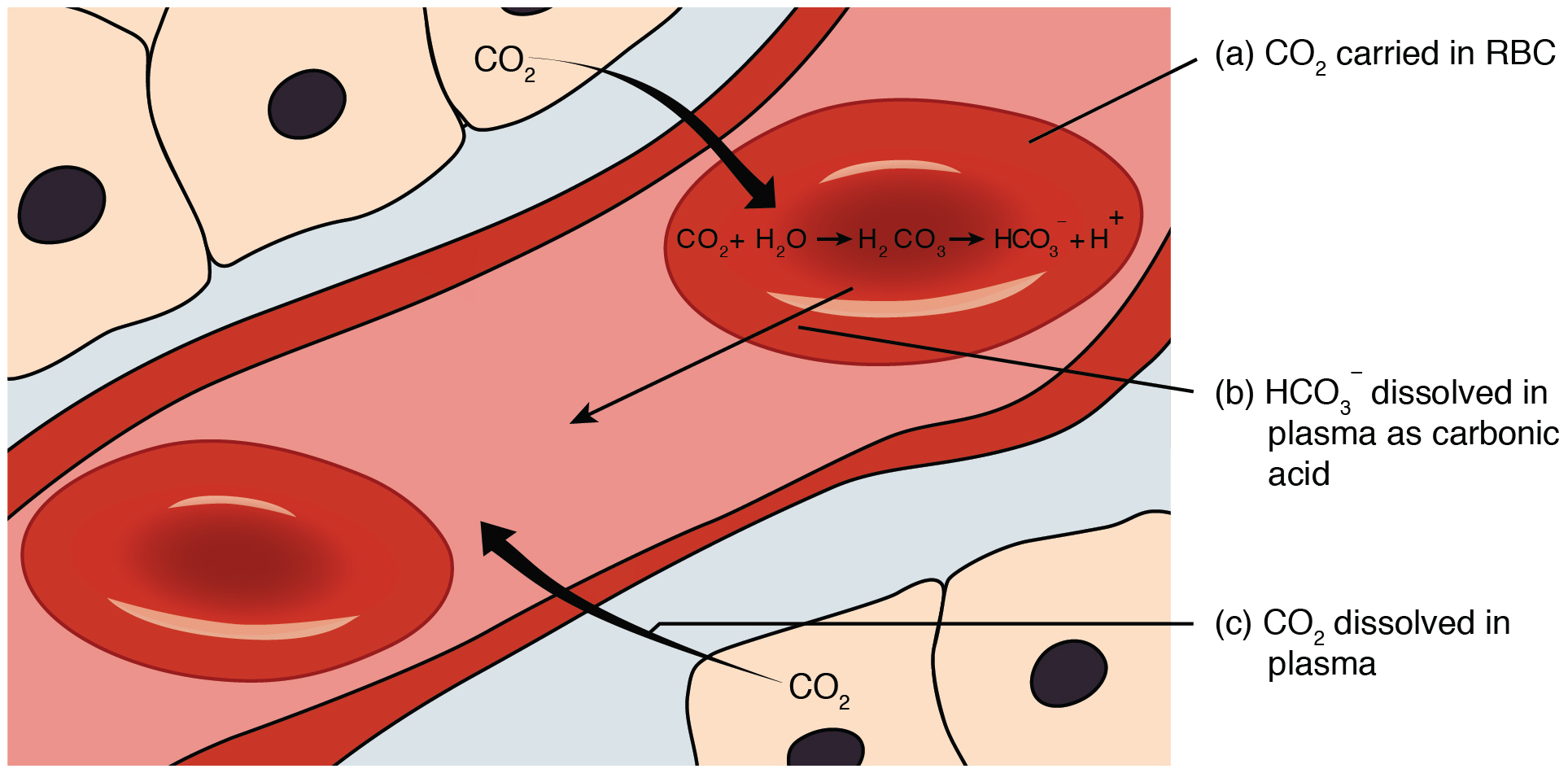
Sodium Bicarbonate To Carbonic Acid. This produces a compound called carbonic acid with the chemical formula H2CO3. Intravenous sodium bicarbonate also known as sodium hydrogen carbonate is a medication primarily used to treat severe metabolic acidosis. Bicarbonate ions also remain in solution. Most of the bicarbonate ions diffuse into the plasma.
 Bicarbonate Buffer System Wikipedia From en.wikipedia.org
Bicarbonate Buffer System Wikipedia From en.wikipedia.org
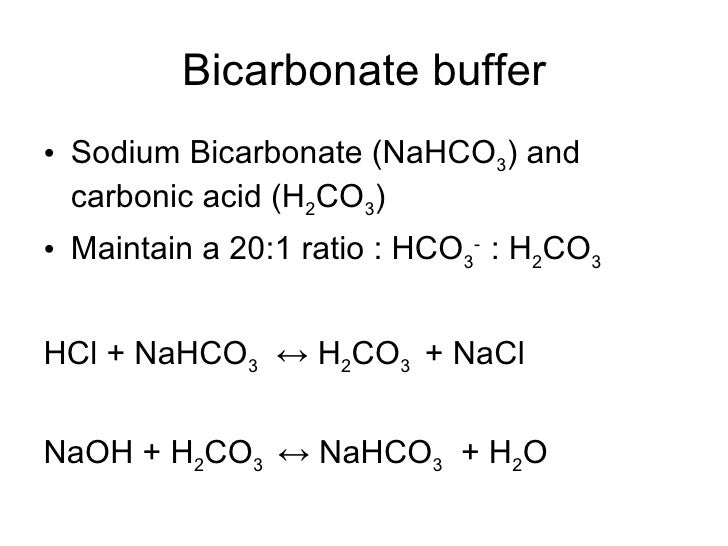
A solution of acetic acid and sodium acetate CH 3 COOH CH 3 COONa is an example of a buffer that consists of a weak acid and its salt. For this purpose it is generally only used when the pH is less than 71 and when the underlying cause is either diarrhea vomiting or the kidneys. 22 to 29 mEqL. H 3 PO 4. It is a white solid. The result of this initial reaction is two new chemicals.
PO 4 3-phosphate.
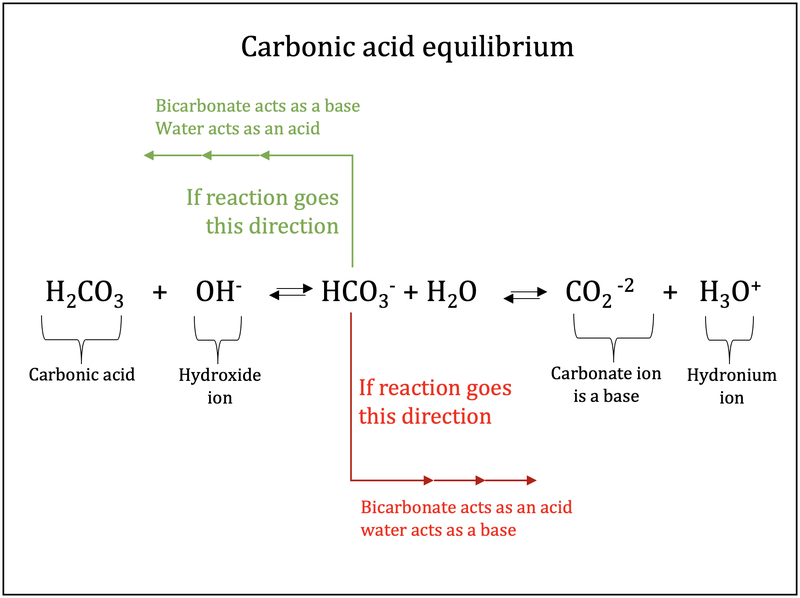
An example of a buffer that consists of a weak base and its salt is a solution of ammonia and ammonium chloride NH 3 aq NH 4 Claq. Since the ratio of H2CO3 to dissolved CO2 is constant at equilibrium pH may be expressed in terms of bicarbonate ion concentration and partial pressure of CO2 by means of the Henderson-Hasselbach equation. The products of weathering are predominantly clays a group of silicate minerals and soluble ions such as calcium iron sodium and potassium. CrO 4 2-chromate. A fire extinguisher containing potassium hydrogencarbonate. Silver nitrate sodium chloride — silver chloride and sodium nitrate.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Bicarbonate de soude carbonate acide de sodium ancien nom sodium bicarbonate médecine NaHCO 3 sodium hydrogen carbonate chimie carbonic acid monosodium salt bicarbonate of soda baking soda ou bread soda anglo-saxon. A solution of acetic acid and sodium acetate CH 3 COOH CH 3 COONa is an example of a buffer that consists of a weak acid and its salt. SO 4 2-sulfate. It is a white solid. The products of weathering are predominantly clays a group of silicate minerals and soluble ions such as calcium iron sodium and potassium.
 Source: socratic.org
Source: socratic.org
The first reaction is the acid-base reaction. BO 3 3-borate. Intravenous sodium bicarbonate also known as sodium hydrogen carbonate is a medication primarily used to treat severe metabolic acidosis. Nursing consideration for Serum Bicarbonate. Silver nitrate sodium chloride — silver chloride and sodium nitrate.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Le bicarbonate de sodium peut être désigné de plusieurs façons selon le contexte ou lépoque. A solution of acetic acid and sodium acetate CH 3 COOH CH 3 COONa is an example of a buffer that consists of a weak acid and its salt. BO 3 3-borate. Mixed with water however the sodium and bicarbonate separate from one another and the bicarbonate reacts with the citric acid. Sodium bicarbonate baking soda vinegar — carbonic acid and sodium acetate.
Source: researchgate.net
A remnant of the carbonic acid. A fire extinguisher containing potassium hydrogencarbonate. H 3 BO 3. Bicarbonate de soude carbonate acide de sodium ancien nom sodium bicarbonate médecine NaHCO 3 sodium hydrogen carbonate chimie carbonic acid monosodium salt bicarbonate of soda baking soda ou bread soda anglo-saxon. A remnant of the carbonic acid.
Source: phe-culturecollections.org.uk
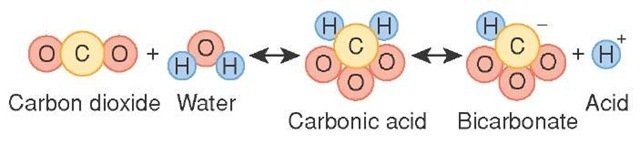
The products of weathering are predominantly clays a group of silicate minerals and soluble ions such as calcium iron sodium and potassium. H 3 PO 4. Carbonic acid disassociates into hydrogen ions and bicarbonate ions. Bicarbonate de soude carbonate acide de sodium ancien nom sodium bicarbonate médecine NaHCO 3 sodium hydrogen carbonate chimie carbonic acid monosodium salt bicarbonate of soda baking soda ou bread soda anglo-saxon. Sodium Bicarbonate sodium bicarbonate 5 injection Injection may be indicated in the treatment of metabolic acidosis which can occur in severe renal disease uncontrolled diabetes circulatory insufficiency due to shock anoxia or severe dehydration extracorporeal circulation of blood and severe primary lactic acidosisSodium Bicarbonate sodium bicarbonate 5 injection.
 Source: cavemanchemistry.com
Source: cavemanchemistry.com
Sulfuric acid barium hydroxide — barium sulfate and water. PH pk log HCO3-aPCO2. When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Sulfuric acid barium hydroxide — barium sulfate and water. The products of weathering are predominantly clays a group of silicate minerals and soluble ions such as calcium iron sodium and potassium.
 Source: slidetodoc.com
Source: slidetodoc.com
It is manufactured by treating an aqueous solution of potassium carbonate with carbon dioxide. The products of weathering are predominantly clays a group of silicate minerals and soluble ions such as calcium iron sodium and potassium. 6351 carbonic acid. Ammonium bicarbonate appears as a white crystalline solid having the odor of ammoniaSoluble in waterThe primary hazard is the threat to the environment. Since the ratio of H2CO3 to dissolved CO2 is constant at equilibrium pH may be expressed in terms of bicarbonate ion concentration and partial pressure of CO2 by means of the Henderson-Hasselbach equation.
 Source: visionlearning.com
Source: visionlearning.com
Nursing consideration for Serum Bicarbonate. You can also cause a double replacement chemical reaction when you combine an acid and a base. CrO 4 2-chromate. Bicarbonate ions also remain in solution. The products of weathering are predominantly clays a group of silicate minerals and soluble ions such as calcium iron sodium and potassium.
 Source: timescavengers.blog
Source: timescavengers.blog
Sodium bicarbonate baking soda vinegar — carbonic acid and sodium acetate. Il est en effet traditionnellement fait du pain au bicarbonate. K 2 CO 3 CO 2 H 2 O. When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. Bicarbonate ions also remain in solution.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The carbonic acid formed dissociates into bicarbonate and hydrogen ions. BO 3 3-borate. Ammonium bicarbonate appears as a white crystalline solid having the odor of ammoniaSoluble in waterThe primary hazard is the threat to the environment. Most of the bicarbonate ions diffuse into the plasma. Sodium bicarbonate baking soda vinegar — carbonic acid and sodium acetate.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium bicarbonate to carbonic acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.