Rubbing alcohol chemical properties
Home » chemical » Rubbing alcohol chemical properties >Rubbing alcohol chemical properties
Rubbing Alcohol Chemical Properties. Isopropyl alcohol IUPAC name propan-2-ol and also called isopropanol or 2-propanol is a colorless flammable chemical compound chemical formula CH 3 CHOHCH 3 with a strong odor. For small fires use carbon dioxide dry chemical dry sand or alcohol-resistant foam. Heres What Happens If You Drink Heavy Water. One of the main weaknesses of white vinegar is that it lacks many of the anti-viral properties of rubbing alcohol meaning that its not effective at stopping the transmission of viruses through surfaces.
 Difference Between Isopropyl Alcohol And Ethyl Alcohol From sciencenotes.org
Difference Between Isopropyl Alcohol And Ethyl Alcohol From sciencenotes.org
Leading the way to materials of the future through chemistry our companys functional resins improve the intrinsic performance of general-purpose resins to a maximum level. However that publication failed to mention the employed rubbing time 18. Rubbing alcohol is either an isopropyl alcohol or an ethanol-based liquid or the comparable British Pharmacopoeia BP defined surgical spirit with isopropyl alcohol products being the most widely availableRubbing alcohol is denatured and undrinkable even if it is ethanol-based due to the bitterants added. Propan-1-ol is the parent member of the class of propan-1-ols that is propane in which a hydrogen of one of the methyl groups is replaced by a hydroxy group. For small fires use carbon dioxide dry chemical dry sand or alcohol-resistant foam. Ethanol is a clear colourless liquid with a characteristic pleasant odour and burning taste.
-1282F -89C Isopropyl Alcohol IUPAC ID.
Isopropyl Alcohol Molar Mass. Health-care facilities with limited resources may not have access to a variety of hospital disinfectants however alcohol and bleach are acceptable chemical disinfectants if used appropriately. It is important to consider that SLF egg masses are laid on many surfaces including rocks trees fence posts and outdoor furniture. Alcohol refers to any chemical having an -OH functional group hydroxyl bound to a saturated carbon atom. Rubbing alcohol is either an isopropyl alcohol or an ethanol-based liquid or the comparable British Pharmacopoeia BP defined surgical spirit with isopropyl alcohol products being the most widely availableRubbing alcohol is denatured and undrinkable even if it is ethanol-based due to the bitterants added. Its time to put that bottle to work.
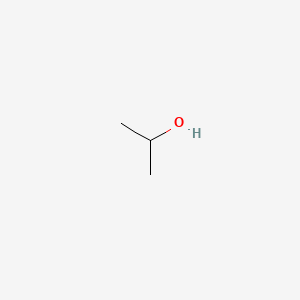
Alcohol is effective against influenza virus. Its time to put that bottle to work. To summarize rubbing alcohol works as a minor cleaning solvent and is meant to be applied as an antiseptic. However that publication failed to mention the employed rubbing time 18. It can decompose into acetone and hydrogen gas according to the following reactionC 3 H 7 OH C 3 H 6 Oacetone.
 Source: haikudeck.com
Source: haikudeck.com
It is a structural isomer of 1-propanol and. In some cases you can substitute one alcohol for another or use a mixture of alcohols. It is highly flammable. 30 Vinegar - 300 Grain Vinegar Concentrate - 1 Gallon of. Isopropyl Alcohol Melting Point.
 Source: haikudeck.com
Source: haikudeck.com
Isopropyl Alcohol Boiling Point. Both are similar enough in their solvent properties that we can generalize. One of the main weaknesses of white vinegar is that it lacks many of the anti-viral properties of rubbing alcohol meaning that its not effective at stopping the transmission of viruses through surfaces. Propan-1-ol is the parent member of the class of propan-1-ols that is propane in which a hydrogen of one of the methyl groups is replaced by a hydroxy group. In some cases you can substitute one alcohol for another or use a mixture of alcohols.
 Source: study.com
Source: study.com
Heres What Happens If You Drink Heavy Water. Isopropyl Alcohol Molar Mass. In some cases you can substitute one alcohol for another or use a mixture of alcohols. They are liquids used primarily as a topical antiseptic. Here are 10 handy household uses for rubbing alcohol.
 Source: chemicals.co.uk
Source: chemicals.co.uk
Do NOT use straight streams of water. It is a structural isomer of 1-propanol and. Rubbing or isopropyl alcohol is a common and surprisingly versatile household item. The Reason Lithium Batteries Catch Fire and Explode. 1805F 825C Isopropyl Alcohol Density.

Isopropyl alcohol CH3CHOHCH3 or CH32CHOH or C3H8O CID 3776 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Isopropyl Alcohol Molar Mass. It is a structural isomer of 1-propanol and. How effectively it works can. It can decompose into acetone and hydrogen gas according to the following reactionC 3 H 7 OH C 3 H 6 Oacetone.

Rubbing alcohol is either an isopropyl alcohol or an ethanol-based liquid or the comparable British Pharmacopoeia BP defined surgical spirit with isopropyl alcohol products being the most widely availableRubbing alcohol is denatured and undrinkable even if it is ethanol-based due to the bitterants added. Alcohol will damage some plastics but not all. The Reason Lithium Batteries Catch Fire and Explode. It reacts in a complex way with bromine giving mainly brominated acetones such as BrCH 2 COCBr 3 and isopropyl bromideC 3 H 7 OH BrCH2COCBr 3 CH 3 CHBrCH 3 isopropyl bromide It is the main ingredient in rubbing alcohol. Leading the way to materials of the future through chemistry our companys functional resins improve the intrinsic performance of general-purpose resins to a maximum level.
 Source: sciencenotes.org
Source: sciencenotes.org
It is a structural isomer of 1-propanol and. Alcohol will damage some plastics but not all. You need it for its antiseptic properties but you can use it lots of other ways. Chemical Properties of Isopropyl Alcohol C 3 H 8 O. However that publication failed to mention the employed rubbing time 18.

Health-care facilities with limited resources may not have access to a variety of hospital disinfectants however alcohol and bleach are acceptable chemical disinfectants if used appropriately. Spray the mixture onto glass mirrors ceramic tiles and chrome finishes then wipe with a paper towel or microfiber cloth. Experimental Melting Point-90 C Alfa Aesar -88 C Jean-Claude Bradley Open Melting Point Dataset 13121-895 C Jean-Claude Bradley Open Melting Point Dataset 16682 21816-90 C Jean-Claude Bradley Open Melting Point Dataset 3050-90 C Alfa Aesar L10181 36644 19397 22906 40983 39194 41840 41463-895 C Oakwood-885 C FooDB. Ethanol is a clear colourless liquid with a characteristic pleasant odour and burning taste. Ethyl alcohol 70 is a powerful broad.
 Source: study.com
Source: study.com
Pour them into a spray bottle. Ethanol is used to dissolve other chemical substances and mixes readily with water and many organic liquids. Health-care facilities with limited resources may not have access to a variety of hospital disinfectants however alcohol and bleach are acceptable chemical disinfectants if used appropriately. It is highly flammable. Easy Ways to Substitute for Baking Powder and Baking Soda.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title rubbing alcohol chemical properties by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.