Properties of phosphorus trichloride
Home » chemical » Properties of phosphorus trichloride >Properties of phosphorus trichloride
Properties Of Phosphorus Trichloride. PS phosphorus monosulfide cation. Help text not available for this section currently History. Elements and Periodic Table History. Learn about the structure of boric acid molecules along with the properties and uses of H3BO3 at BYJUS.
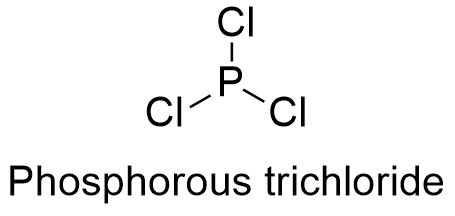 Phosphorous Trichloride Formula From softschools.com
Phosphorous Trichloride Formula From softschools.com
H 2 hydrogen. Each allotrope has different physical properties. Three chlorine atoms and one phosphorus atom make phosphorus trichloridePCl 3 compounds by sharing electrons. Phosphoryl chloride commonly called phosphorus oxychloride is a colourless liquid with the formula The template Phosphorus is being considered for deletion P O The template Chlorine is being considered for deletion Cl 3It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chlorideIt is manufactured industrially on a large scale from phosphorus trichloride. O 3 ozone. 10 times the heat of evaporation keeps the system at its boiling point and the phosphorus trichloride distills off.
O 2 diatomic oxygen cation.
Phosphorus atoms form bonds by sharing electrons with chlorine atoms. H 2 O water. Impure or amorphous boron can be prepared by heating the trioxide with magnesium powder. Carbohydrates sugars and starch Lipids fats Nucleic acids DNA and RNA Note that organic compounds are all covalent compounds. High-purity boron is prepared by reducing boron trichloride or tribromide with hydrogen on electrically heated filaments. Forms explosive nitrogen trichloride from biuret contaminated with cyanuric acid.
 Source: slideshare.net
Source: slideshare.net
In general if a molecule. A colorless liquid when pure it is an important industrial chemical being used for the manufacture of phosphites and other organophosphorus compounds. CH 4 methane. P Cl 2 PCl 3. Insoluble in water and ethyl alcoholSoluble in carbon disulfideShipped as a solid or liquid in an atmosphere of inert gas or as a solid under waterBarely soluble in water and denser than waterHence sinks in waterUses include munitions manufacture pyrotechnics.
 Source: youtube.com
Source: youtube.com
The Exception to the Rule. P4 s 6Cl2 g - 4 PCL3 l what is the maximum amount of phosphorus trichloride that can be formed if 10 molecules of P4 are mixed with 36 molecules of chlorine. The Exception to the Rule. Phosphorus white dry or under water or in solution appears as a soft waxy solid with a sharp pungent odor similar to garlic. In general if a molecule.
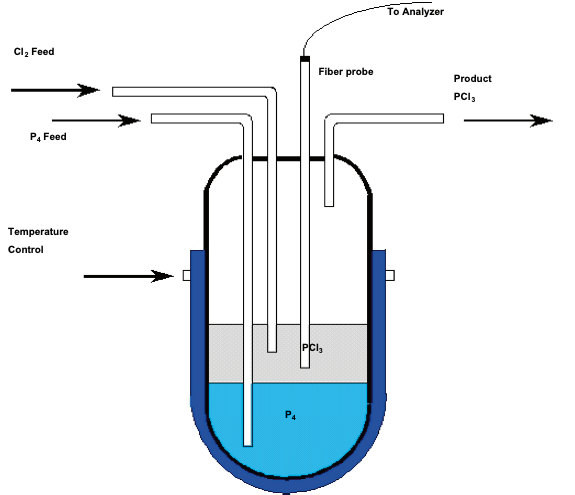 Source: azom.com
Source: azom.com
Ignites as a liquid synthetic and natural rubber. For centuries the only source of borax Na 2 B 2 O 5 OH 4 was the crystallized. Carbohydrates sugars and starch Lipids fats Nucleic acids DNA and RNA Note that organic compounds are all covalent compounds. Help text not available for this section currently History. 10 times the heat of evaporation keeps the system at its boiling point and the phosphorus trichloride distills off.
 Source: toppr.com
Source: toppr.com
CO 2 carbon dioxide. The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorusThe heat of reaction ca. For centuries the only source of borax Na 2 B 2 O 5 OH 4 was the crystallized. HCl hydrogen chloride. NH 3 ammonia.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorusThe heat of reaction ca. O 2 diatomic oxygen cation. For more information on the Visual Elements image see the Uses and properties section below. Ignites or explodes with arsine phosphine silane diborane stibine red phosphorus white phosphorus boron active carbon silicon arsenic. Hennig Brandt Origin of the name.
 Source: softschools.com
Source: softschools.com
Forms explosive nitrogen trichloride from biuret contaminated with cyanuric acid. PCl 3 phosphorus trichloride. The electron configuration of chlorine shows that the valence electrons of chlorine are seven. Hennig Brandt Origin of the name. For more information on the Visual Elements image see the Uses and properties section below.
 Source: unacademy.com
Source: unacademy.com
Readily forms an explosive N-chloro derivative with aziridine. White P Red P Black P P 2 P Phosphorus 15. A colorless liquid when pure it is an important industrial chemical being used for the manufacture of phosphites and other organophosphorus compounds. Phosphorus trichloride is a inorganic compound with the chemical formula PCl 3. It is toxic and reacts violently with water to release hydrogen chloride.
 Source: byjus.com
Source: byjus.com
Boric Acid H3BO3 - Boric acid also called hydrogen borate boracic acid and orthoboric acid is a weak monobasic Lewis acid of boron. Impure or amorphous boron can be prepared by heating the trioxide with magnesium powder. 10 times the heat of evaporation keeps the system at its boiling point and the phosphorus trichloride distills off. Phosphorus trichloride is a inorganic compound with the chemical formula PCl 3. CO 2 carbon dioxide.
 Source: studylib.net
Source: studylib.net
Readily forms an explosive N-chloro derivative with aziridine. CO 2 carbon dioxide. P4 s 6Cl2 g - 4 PCL3 l what is the maximum amount of phosphorus trichloride that can be formed if 10 molecules of P4 are mixed with 36 molecules of chlorine. S 2 sulfur diatomic cation. The phosphorus-containing cationic exchange resins are prepared by the treatment of three-dimensional polymers of unsaturated aromatic hydrocarbons with phosphorus trichloride in the presence of aluminium chloride or by the treatment of polymers containing halogenated methyl groups with trialkyl phosphates followed by oxidation and hydrolysis of the reaction products 517 518 521 524.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Hennig Brandt Origin of the name. CH 3 CH 2 OH ethanol. Hennig Brandt Origin of the name. PO phosphorus monoxide cation. Identify the diprotic acid.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title properties of phosphorus trichloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.