Properties of hydrogen sulfide
Home » chemical » Properties of hydrogen sulfide >Properties of hydrogen sulfide
Properties Of Hydrogen Sulfide. Physical Properties of hydrogen peroxide. Specific Heat Capacity. Use and re-distribution of the data in whole or in part for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material HMDB and the original publication see the HMDB citing page. Hydrogen is not toxic but is a simple asphyxiate by the displacement of oxygen in the air.
 Hydrogen Sulfide Gas Osha 1910 1000 Ansi Z From slidetodoc.com
Hydrogen Sulfide Gas Osha 1910 1000 Ansi Z From slidetodoc.com
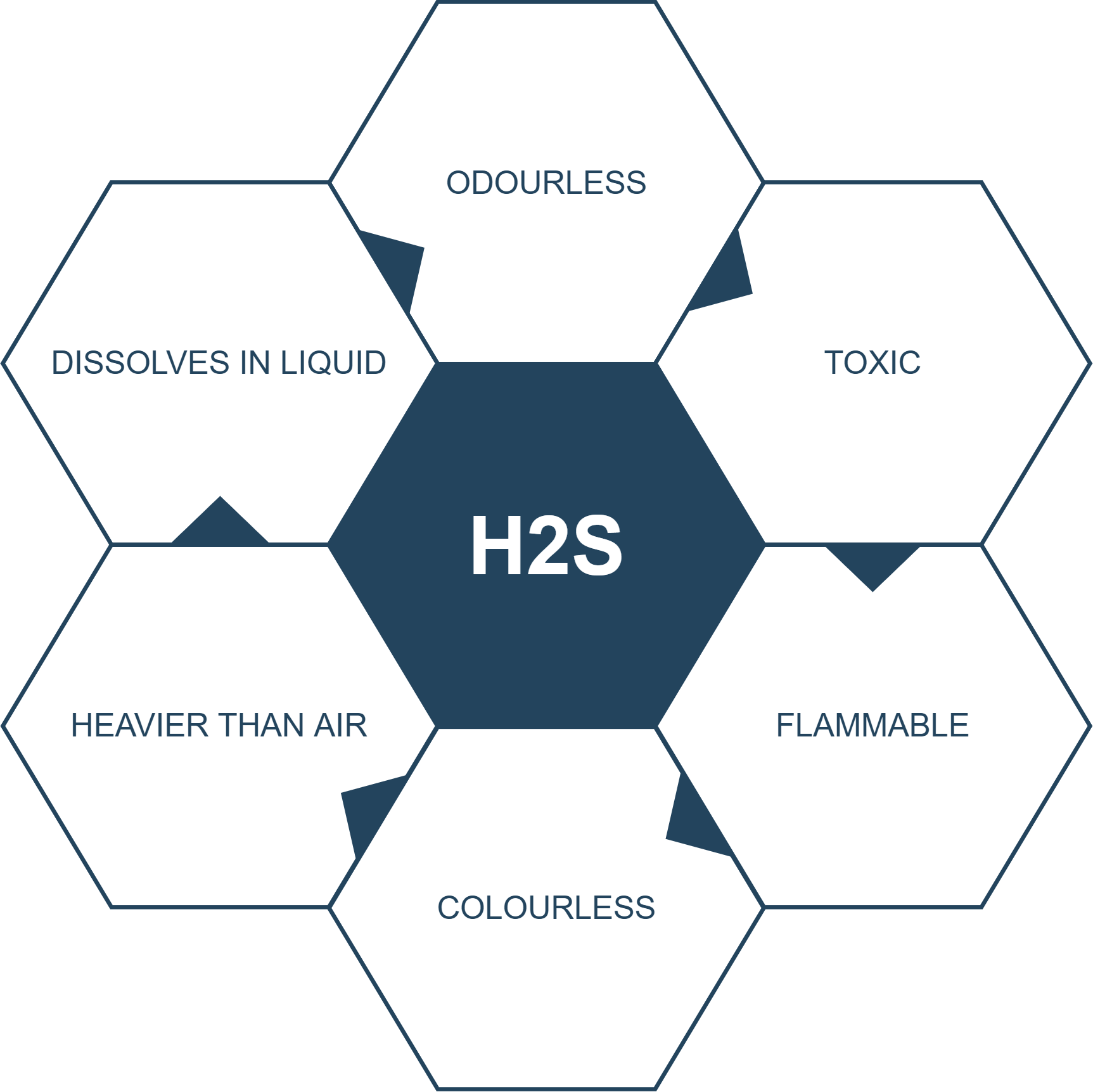
At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2. Sterile inlet connection diam. Hydrogen is the chemical element with the symbol H and atomic number 1. Stars such as the Sun are. Hydrogen sulfide is slightly denser than air. In addition to exposure to hydrogen sulfide in the air exposure to liquid hydrogen sulfide can cause blue skin or frostbite.
If clothing becomes wet avoid.
Hydrogen is the lightest element. If clothing becomes wet avoid. The subsequent step is the oxidation of adsorbed hydrogen sulfide to elemental sulfur in the presence of dissolved oxygen. A mixture of H 2 S and air can be explosive. When at solid state hydrogen peroxide is a white crystal. When exposes to the sunlight hydrogen peroxide decomposes to oxygen gas and water.
 Source: semanticscholar.org
Source: semanticscholar.org
If clothing becomes wet avoid. It is flammable over a wide range of vaporair concentrations. The vapors are lighter than air. In this capacity catalytic carbon. Has weak acidic characteristics.
 Source: galnet.fandom.com
Source: galnet.fandom.com
Every substance has its own specific heat capacity with the specific heat capacity of water being 1 calgC. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. The specific heat capacity of water is much higher than that of other common. 916 in pore size 02 μm pore size 05 μm cartridge nominal length 3 in. Specific Heat Capacity.
 Source: scotdir.com
Source: scotdir.com
Hydrogen is the chemical element with the symbol H and atomic number 1. The vapors are lighter than air. Stars such as the Sun are. 916 in pore size 02 μm pore size 05 μm cartridge nominal length 3 in. In addition to exposure to hydrogen sulfide in the air exposure to liquid hydrogen sulfide can cause blue skin or frostbite.
 Source: byjus.com
Source: byjus.com
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the chemical element with the symbol H and atomic number 1. The specific heat capacity of water is much higher than that of other common. The subsequent step is the oxidation of adsorbed hydrogen sulfide to elemental sulfur in the presence of dissolved oxygen. Hydrogen is not toxic but is a simple asphyxiate by the displacement of oxygen in the air.
 Source: researchgate.net
Source: researchgate.net
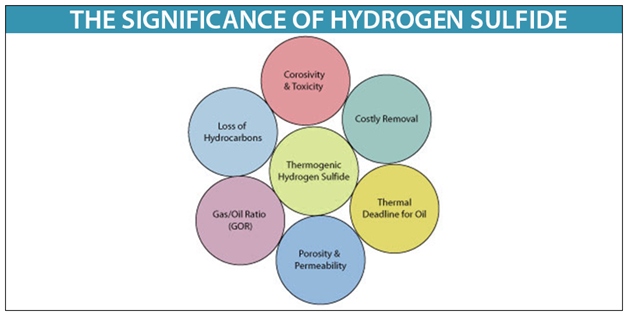
Hydrogen H2 is a colorless odorless gas. Hydrogen sulfide burns in oxygen with a blue flame to form sulfur dioxide SO 2 and water. When at solid state hydrogen peroxide is a white crystal. Hydrogen sulfide is a highly flammable explosive gas and can cause possible life-threatening situations if not properly handled. The specific heat capacity of water is much higher than that of other common.
 Source: slidetodoc.com
Source: slidetodoc.com
Hydrogen sulfide is a highly flammable explosive gas and can cause possible life-threatening situations if not properly handled. HMDB is offered to the public as a freely available resource. Hydrogen H2 is a colorless odorless gas. In addition hydrogen sulfide gas burns and produces other toxic vapors and gases such as sulfur dioxide. The subsequent step is the oxidation of adsorbed hydrogen sulfide to elemental sulfur in the presence of dissolved oxygen.
 Source: instrumentationbasic.com
Source: instrumentationbasic.com
Physical Properties of hydrogen peroxide. In general hydrogen sulfide acts as a reducing agent especially in the presence of base which forms SH. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. When hydrogen peroxide exists as pure viscous at liquid state it is colourless. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2.
 Source: slideplayer.com
Source: slideplayer.com
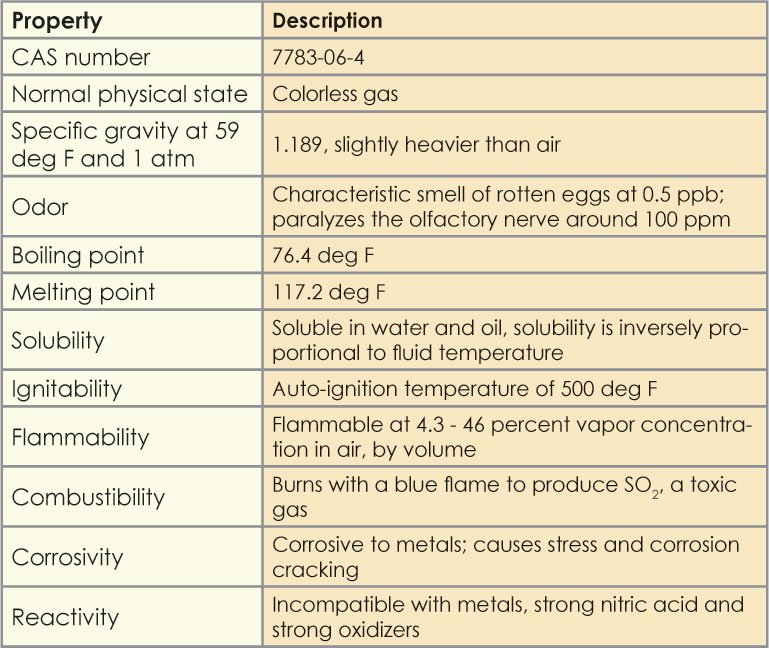
Hydrogen is not toxic but is a simple asphyxiate by the displacement of oxygen in the air. Every substance has its own specific heat capacity with the specific heat capacity of water being 1 calgC. In addition hydrogen sulfide gas burns and produces other toxic vapors and gases such as sulfur dioxide. Fast Facts provides physical properties such as molecular weight critical temperature and pressure liquid and gas density and specific gravity. Sterile inlet connection diam.
 Source: drillingcontractor.org
Source: drillingcontractor.org
Also at higher temperature H 2 O 2 may be explosive. Catalytic carbon has all of the adsorptive properties of conventional GAC but it can also convert hydrogen sulfide to elemental sulfur. Physical Properties of hydrogen peroxide. Specific Heat Capacity. Use and re-distribution of the data in whole or in part for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material HMDB and the original publication see the HMDB citing page.
 Source: slideshare.net
Source: slideshare.net
Once ignited it burns with a pale blue almost invisible flame. Hydrogen sulfide is slightly denser than air. When at solid state hydrogen peroxide is a white crystal. The specific heat capacity of water is much higher than that of other common. Once ignited it burns with a pale blue almost invisible flame.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title properties of hydrogen sulfide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.