Properties of barium
Home » chemical » Properties of barium >Properties of barium
Properties Of Barium. At 910C it changes to γ-iron which is much softer in nature. The table below gives the name atomic number electronic configuration of the atom the first second and third ionisation energy melting point density and electronegativity of the Group 2 elements alkaline-earth. It rusts in damp air but not in the dry air. Barium nitrate BaNO32 or BaN2O6 CID 24798 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
 Barium Ba Element 56 Of Periodic Table Elements Flashcards From newtondesk.com
Barium Ba Element 56 Of Periodic Table Elements Flashcards From newtondesk.com
The table below gives the name atomic number electronic configuration of the atom the first second and third ionisation energy melting point density and electronegativity of the Group 2 elements alkaline-earth. Paramagnetic Polyethylene Spheres Black paramagnetic microspheres in dry powder form have enabled scientists to leverage the benefits of paramagnetic particles in new applications. Barium is a soft silvery-white metal with a slight golden shade when ultrapure. It rusts in damp air but not in the dry air. At room temperature this metal is in the form of ferrite or α-form. No ads no money for us no free stuff for you.
Barium is a soft silvery-white metal with a slight golden shade when ultrapure.
At 910C it changes to γ-iron which is much softer in nature. At 0 C 558 g of barium acetate can be dissolved in 100 g of water. Paramagnetic Polyethylene Spheres Black paramagnetic microspheres in dry powder form have enabled scientists to leverage the benefits of paramagnetic particles in new applications. Physical Properties of Iron. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Please do not block ads on this website.
 Source:
Source:
Barium acetate is a white powder which is highly soluble. At 0 C 558 g of barium acetate can be dissolved in 100 g of water. The table below gives the name atomic number electronic configuration of the atom the first second and third ionisation energy melting point density and electronegativity of the Group 2 elements alkaline-earth. It decomposes upon heating into barium carbonate. When heated in air barium acetate decomposes to the carbonate.
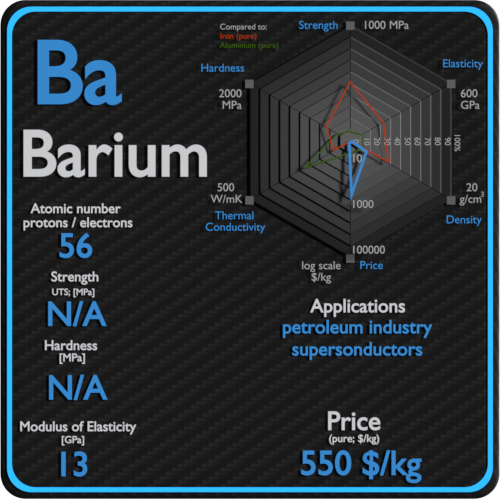 Source: material-properties.org
Source: material-properties.org
Table of Data for Group 2 Elements. The table below gives the name atomic number electronic configuration of the atom the first second and third ionisation energy melting point density and electronegativity of the Group 2 elements alkaline-earth. Barium is a soft silvery-white metal with a slight golden shade when ultrapure. It melts at 1536C and boils at 2861C. It decomposes upon heating into barium carbonate.
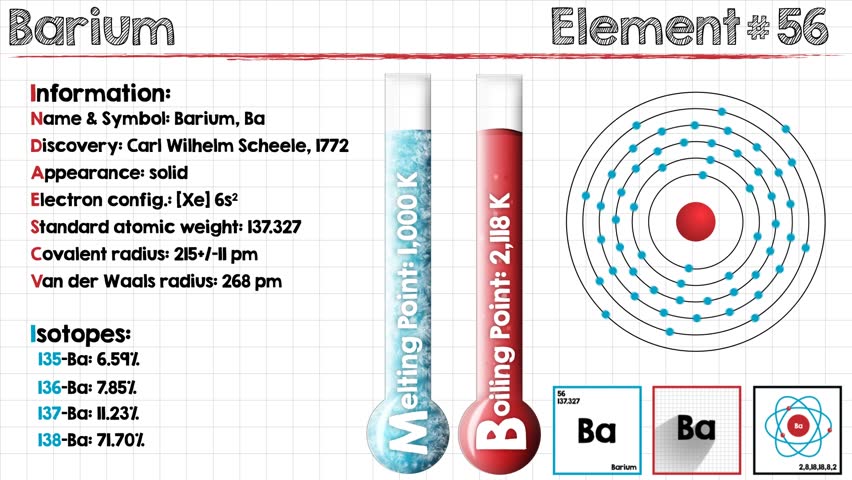 Source: shutterstock.com
Source: shutterstock.com
At 0 C 558 g of barium acetate can be dissolved in 100 g of water. Barium Chloride BaCl2 - Barium Chloride is an inorganic compound with the chemical formula BaCl2. It decomposes upon heating into barium carbonate. Barium sulfate contrast material is embedded into the polyethylene microspheres and acts as a radiocontrast agent for diagnostic procedures. When heated in air barium acetate decomposes to the carbonate.
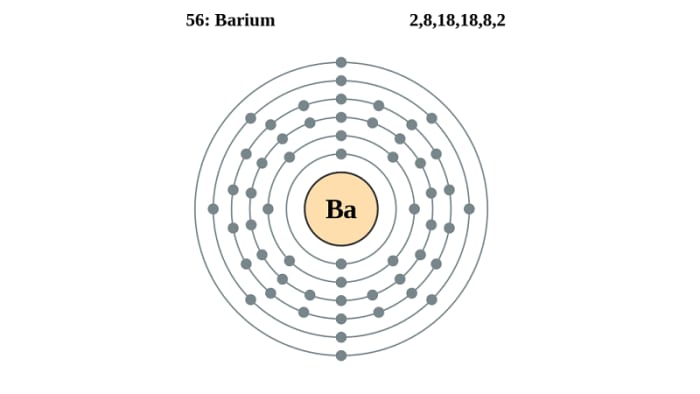 Source: melscience.com
Source: melscience.com
When heated in air barium acetate decomposes to the carbonate. Please do not block ads on this website. When heated in air barium acetate decomposes to the carbonate. Physical Properties of Iron. Barium is a soft silvery-white metal with a slight golden shade when ultrapure.
 Source: britannica.com
Source: britannica.com
At room temperature this metal is in the form of ferrite or α-form. No ads no money for us no free stuff for you. It melts at 1536C and boils at 2861C. It dissolves readily in dilute acids. Barium sulfate contrast material is embedded into the polyethylene microspheres and acts as a radiocontrast agent for diagnostic procedures.
 Source: britannica.com
Source: britannica.com
2 The silvery-white color of barium metal rapidly vanishes upon oxidation in air yielding a dark gray layer containing the oxideBarium has a medium specific weight and high electrical conductivity. Barium is a soft silvery-white metal with a slight golden shade when ultrapure. Because barium is difficult to purify many of its properties have not been accurately determined. When heated in air barium acetate decomposes to the carbonate. Paramagnetic Polyethylene Spheres Black paramagnetic microspheres in dry powder form have enabled scientists to leverage the benefits of paramagnetic particles in new applications.
 Source: newtondesk.com
Source: newtondesk.com
At 0 C 558 g of barium acetate can be dissolved in 100 g of water. Learn about the uses physical chemical properties and the structure of Barium Chloride BaCl2. No ads no money for us no free stuff for you. At 0 C 558 g of barium acetate can be dissolved in 100 g of water. It dissolves readily in dilute acids.
 Source: slideshare.net
Source: slideshare.net
It decomposes upon heating into barium carbonate. Table of Data for Group 2 Elements. At 0 C 558 g of barium acetate can be dissolved in 100 g of water. At 910C it changes to γ-iron which is much softer in nature. At room temperature this metal is in the form of ferrite or α-form.
 Source: freechemistryonline.com
Source: freechemistryonline.com
Please do not block ads on this website. 2 The silvery-white color of barium metal rapidly vanishes upon oxidation in air yielding a dark gray layer containing the oxideBarium has a medium specific weight and high electrical conductivity. Barium Chloride BaCl2 - Barium Chloride is an inorganic compound with the chemical formula BaCl2. Because barium is difficult to purify many of its properties have not been accurately determined. At 910C it changes to γ-iron which is much softer in nature.
 Source: melscience.com
Source: melscience.com
At 910C it changes to γ-iron which is much softer in nature. When heated in air barium acetate decomposes to the carbonate. Barium Chloride BaCl2 - Barium Chloride is an inorganic compound with the chemical formula BaCl2. Being a metal is magnetic in nature. At 0 C 558 g of barium acetate can be dissolved in 100 g of water.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title properties of barium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.