Properties of ammonium chloride
Home » chemical » Properties of ammonium chloride >Properties of ammonium chloride
Properties Of Ammonium Chloride. 36208 gmol Appearance liquid. Carnitine can be synthesized from the amino acids lysine and. Quaternary ammonium compound l-carnitine 3-hydroxy-4-N-trimethylammonio-butanoate has been shown to possess free-radical scavenging and antioxidant properties and was found to ameliorate oxidative organ injury in various pathologic conditions such as coronary heart disease heart or renal failure and ischemia-reperfusion 3848. Except where otherwise noted data are given for materials in their standard state at 25 C 77.
 Amonium Chloride From pt.slideshare.net
Amonium Chloride From pt.slideshare.net
Tetrabutylammonium chloride C16H36ClN CID 70681 - structure chemical names physical and chemical properties classification patents literature biological. 087 gcm 3 20 C Pharmacology ATC code. In patients with normal hepatic function the ammonium cation is converted to urea by the liver and a hydrogen cation is released which reacts with a bicarbonate ion to form water and carbon dioxide. The solid dissolves readily in water and its solutions have a salt-like tastePotassium chloride can be obtained from ancient dried lake deposits. The most important of such. Didecyl dimethyl ammonium chloride.
Tetrabutylammonium chloride C16H36ClN CID 70681 - structure chemical names physical and chemical properties classification patents literature biological.
The most important of such. Didecyl dimethyl ammonium chloride. D08AJ06 Hazards Main hazards. Except where otherwise noted data are given for materials in their standard state at 25 C 77. The acid-forming properties of ammonium chloride result from dissociation of the salt to an ammonium cation and a chloride anion. Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorineIt is odorless and has a white or colorless vitreous crystal appearance.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorineIt is odorless and has a white or colorless vitreous crystal appearance. The chloride anion combines with fixed bases in the extracellular. In patients with normal hepatic function the ammonium cation is converted to urea by the liver and a hydrogen cation is released which reacts with a bicarbonate ion to form water and carbon dioxide. M can be the cation of sodium potassium rubidium cesium ammonium or thallium and the aluminum may be replaced by a variety of other M 3 ionseg gallium indium titanium vanadium chromium manganese iron or cobalt. Except where otherwise noted data are given for materials in their standard state at 25 C 77.
 Source: freechemistryonline.com
Source: freechemistryonline.com
Didecyl dimethyl ammonium chloride. Quaternary ammonium compound l-carnitine 3-hydroxy-4-N-trimethylammonio-butanoate has been shown to possess free-radical scavenging and antioxidant properties and was found to ameliorate oxidative organ injury in various pathologic conditions such as coronary heart disease heart or renal failure and ischemia-reperfusion 3848. D08AJ06 Hazards Main hazards. 087 gcm 3 20 C Pharmacology ATC code. M can be the cation of sodium potassium rubidium cesium ammonium or thallium and the aluminum may be replaced by a variety of other M 3 ionseg gallium indium titanium vanadium chromium manganese iron or cobalt.
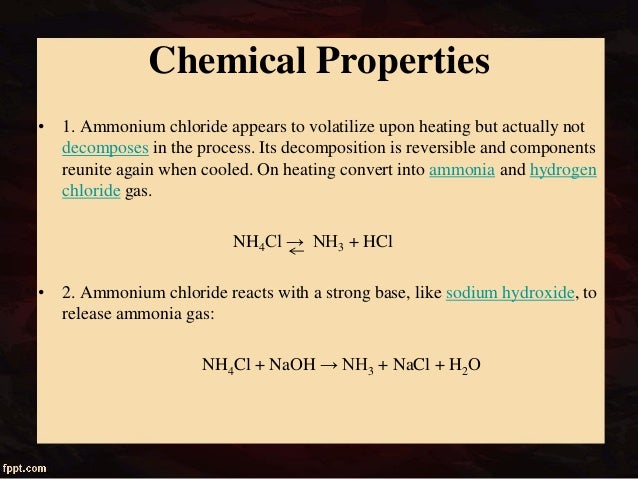 Source: pt.slideshare.net
Source: pt.slideshare.net
Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorineIt is odorless and has a white or colorless vitreous crystal appearance. KCl is used as a fertilizer in medicine in scientific applications and. Tetrabutylammonium chloride C16H36ClN CID 70681 - structure chemical names physical and chemical properties classification patents literature biological. Except where otherwise noted data are given for materials in their standard state at 25 C 77. Carnitine can be synthesized from the amino acids lysine and.
 Source: researchgate.net
Source: researchgate.net
The solid dissolves readily in water and its solutions have a salt-like tastePotassium chloride can be obtained from ancient dried lake deposits. Didecyl dimethyl ammonium chloride. In patients with normal hepatic function the ammonium cation is converted to urea by the liver and a hydrogen cation is released which reacts with a bicarbonate ion to form water and carbon dioxide. Carnitine can be synthesized from the amino acids lysine and. 36208 gmol Appearance liquid.
 Source: slideplayer.com
Source: slideplayer.com
Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorineIt is odorless and has a white or colorless vitreous crystal appearance. The alums double salts of formula MAlSO 4 2 12H 2 O where M is a singly charged cation such as K also contain the Al 3 ion. Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorineIt is odorless and has a white or colorless vitreous crystal appearance. Didecyl dimethyl ammonium chloride. Carnitine can be synthesized from the amino acids lysine and.
 Source: youtube.com
Source: youtube.com
The solid dissolves readily in water and its solutions have a salt-like tastePotassium chloride can be obtained from ancient dried lake deposits. 087 gcm 3 20 C Pharmacology ATC code. Tetrabutylammonium chloride C16H36ClN CID 70681 - structure chemical names physical and chemical properties classification patents literature biological. KCl is used as a fertilizer in medicine in scientific applications and. Quaternary ammonium compound l-carnitine 3-hydroxy-4-N-trimethylammonio-butanoate has been shown to possess free-radical scavenging and antioxidant properties and was found to ameliorate oxidative organ injury in various pathologic conditions such as coronary heart disease heart or renal failure and ischemia-reperfusion 3848.
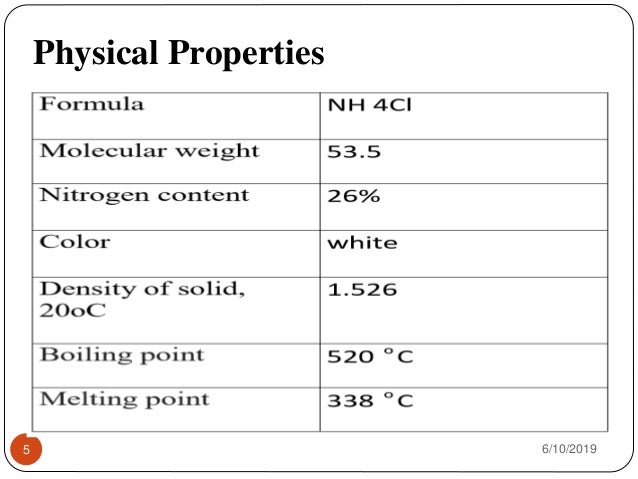 Source: slideshare.net
Source: slideshare.net
Carnitine can be synthesized from the amino acids lysine and. C 22 H 48 ClN Molar mass. In patients with normal hepatic function the ammonium cation is converted to urea by the liver and a hydrogen cation is released which reacts with a bicarbonate ion to form water and carbon dioxide. KCl is used as a fertilizer in medicine in scientific applications and. Quaternary ammonium compound l-carnitine 3-hydroxy-4-N-trimethylammonio-butanoate has been shown to possess free-radical scavenging and antioxidant properties and was found to ameliorate oxidative organ injury in various pathologic conditions such as coronary heart disease heart or renal failure and ischemia-reperfusion 3848.
 Source: freechemistryonline.com
Source: freechemistryonline.com
Tetrabutylammonium chloride C16H36ClN CID 70681 - structure chemical names physical and chemical properties classification patents literature biological. Except where otherwise noted data are given for materials in their standard state at 25 C 77. The alums double salts of formula MAlSO 4 2 12H 2 O where M is a singly charged cation such as K also contain the Al 3 ion. Didecyl dimethyl ammonium chloride. Quaternary ammonium compound l-carnitine 3-hydroxy-4-N-trimethylammonio-butanoate has been shown to possess free-radical scavenging and antioxidant properties and was found to ameliorate oxidative organ injury in various pathologic conditions such as coronary heart disease heart or renal failure and ischemia-reperfusion 3848.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The most important of such. 36208 gmol Appearance liquid. We would like to show you a description here but the site wont allow us. Quaternary ammonium compound l-carnitine 3-hydroxy-4-N-trimethylammonio-butanoate has been shown to possess free-radical scavenging and antioxidant properties and was found to ameliorate oxidative organ injury in various pathologic conditions such as coronary heart disease heart or renal failure and ischemia-reperfusion 3848. The acid-forming properties of ammonium chloride result from dissociation of the salt to an ammonium cation and a chloride anion.
 Source: toppr.com
Source: toppr.com
The chloride anion combines with fixed bases in the extracellular. Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorineIt is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water and its solutions have a salt-like tastePotassium chloride can be obtained from ancient dried lake deposits. 36208 gmol Appearance liquid. The alums double salts of formula MAlSO 4 2 12H 2 O where M is a singly charged cation such as K also contain the Al 3 ion.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title properties of ammonium chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.