Potassium dihydrogen phosphate
Home » chemical » Potassium dihydrogen phosphate >Potassium dihydrogen phosphate
Potassium Dihydrogen Phosphate. Water H 2 O is constantly breaking up to form trace amounts of hydroxide OH- and hydronium H 3 O. Potassium K 1 bromide Br 1 thiosulfate S 2 O 3 2 2 A note about hydrogen and hydronium. The -ite ending indicates a low. Our purpose is to solve the toughest problems in life science by collaborating with the global scientific community and through that we aim to accelerate access to better health for people everywhere.
 Cas 7778 77 0 Sigma Aldrich From sigmaaldrich.com
Cas 7778 77 0 Sigma Aldrich From sigmaaldrich.com
Mono-Potassium orthophosphate Potassium biphosphate Potassium phosphate monobasic CAS. 2. Potassium dihydrogen phosphate the potassium salt is useful to human in the form of pesticides. With e-car and e-bicycle use for internal transport GSFC has further invested in not just the green present but also a greener future. 1 Structures Expand this section. The -ite ending indicates a low.
Also keep in mind phosphate may.
It is a source of phosphorus and potassium as well as a buffering agent. Potassium dihydrogen phosphate is a fungicide that is used to prevent powdery mildew on many fruits. The -ite ending indicates a low. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. Dissolve 340 g of potassium dihydrogen phosphate and 355 g of anhydrous disodium hydrogen phosphate both previously dried at 110 to 130 for 2 hours in sufficient water to produce 1000 ml. Polyatomic ions are ions which consist of more than one atom.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Potassium Ca2 Rb rubidium Sr2 strontium Cs cesium Ba2 calcium barium Fr francium Ra2 radium B boron C carbon nitride N3-oxide O2-fluoride F-neon Ne Al3 aluminum Si silicon phosphide P3-sulfide S2-chloride Cl-argon Ar helium He Zn2 zinc In3 indium Ge4 germanium As3-arsenide selenide Se2-bromide Br-krypton Kr telluride Te2-Po2 poloniumII poloniumIV Po4 iodide I-xenon Xe. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. FDC colors All G. When H is written in equations or textbooks it usually is a simplified way of saying H 3 O.
 Source: fishersci.se
Source: fishersci.se
At first glance the nomenclature of the polyatomic negative ions in the table above seems hopeless. FDC colors All G. Polyfusor NA contains Na 162 mmollitre K 19 mmollitre PO 4 3-100 mmollitre. Please note that the products mentioned below may not be available in all countries. Potassium Ca2 Rb rubidium Sr2 strontium Cs cesium Ba2 calcium barium Fr francium Ra2 radium B boron C carbon nitride N3-oxide O2-fluoride F-neon Ne Al3 aluminum Si silicon phosphide P3-sulfide S2-chloride Cl-argon Ar helium He Zn2 zinc In3 indium Ge4 germanium As3-arsenide selenide Se2-bromide Br-krypton Kr telluride Te2-Po2 poloniumII poloniumIV Po4 iodide I-xenon Xe.
 Source: merckmillipore.com
Source: merckmillipore.com
Dissolve 68 g of potassium dihydrogen orthophosphate in sufficient water to produce l000ml. EDTA may also be added in cellular preparation to prevent clumping. Phosphate Buffer 005 M. Gomori buffers the most commonly used phosphate buffers consist of a mixture of monobasic dihydrogen phosphate and dibasic monohydrogen phosphate. Fruits that can benefit from the addition of potassium dihydrogen phosphate includes common fruits peppers and roses.
 Source: uk.vwr.com
Source: uk.vwr.com
A stock solution of buffered dilution water is prepared by dissolving 340 g of potassium dihydrogen phosphate KH 2PO 4 in 500 ml of distilled water. Dissolve 25 g. The pH is checked and if. The name of the ion usually ends in either -ite or -ate. Phosphate Buffer 005 M.
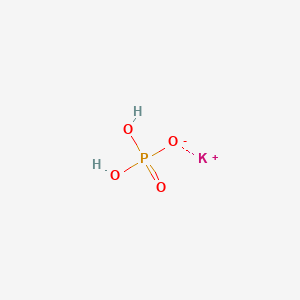
With e-car and e-bicycle use for internal transport GSFC has further invested in not just the green present but also a greener future. The other oxide of hydrogen is H2O2. Water H 2 O is constantly breaking up to form trace amounts of hydroxide OH- and hydronium H 3 O. 2 Names and Identifiers Expand this section. Gomori buffers the most commonly used phosphate buffers consist of a mixture of monobasic dihydrogen phosphate and dibasic monohydrogen phosphate.
 Source: ebay.co.uk
Source: ebay.co.uk
Dissolve 68 g of potassium dihydrogen orthophosphate in sufficient water to produce l000ml. Water H 2 O is constantly breaking up to form trace amounts of hydroxide OH- and hydronium H 3 O. Phosphate Buffer 005 M. Ions grouped by charge anions grouped by periodic position Simple ions. CID 313 Hydrochloric acid CID 1004 Phosphoric acid CID 5360545 Sodium CID 5462222 Potassium Dates.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Additionally currently approved indications uses and presentations may differ between countries. Potassium Ca2 Rb rubidium Sr2 strontium Cs cesium Ba2 calcium barium Fr francium Ra2 radium B boron C carbon nitride N3-oxide O2-fluoride F-neon Ne Al3 aluminum Si silicon phosphide P3-sulfide S2-chloride Cl-argon Ar helium He Zn2 zinc In3 indium Ge4 germanium As3-arsenide selenide Se2-bromide Br-krypton Kr telluride Te2-Po2 poloniumII poloniumIV Po4 iodide I-xenon Xe. Hexa- hecta and hepta-esters of sucrose. By varying the amount of each salt a range of buffers can be prepared that buffer well between pH 58 and pH 80 please see the tables below. It can be used in fertilizer mixtures to reduce escape of ammonia by keeping pH low.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Sodium chloride-disodium hydrogen phosphate-potassium chloride-potassium dihydrogen phosphate 1111 Molecular Weight. There are several general rules however that can bring some order out of this apparent chaos. Also keep in mind phosphate may. AsO 3 3-Hydrogen phosphate. PBS is a water-based salt solution containing sodium hydrogen phosphate sodium chloride and in some cases potassium chloride and potassium dihydrogen phosphate.
 Source: uk.vwr.com
Source: uk.vwr.com
AsO 3 3-Hydrogen phosphate. Water H 2 O is constantly breaking up to form trace amounts of hydroxide OH- and hydronium H 3 O. O2 oxygen F2 fluorine Cl2 chlorine Br2 bromine I2. It is a source of phosphorus and potassium as well as a buffering agent. AsO 3 3-Hydrogen phosphate.
 Source: glentham.com
Source: glentham.com
O2 oxygen F2 fluorine Cl2 chlorine Br2 bromine I2. Potassium Ca2 Rb rubidium Sr2 strontium Cs cesium Ba2 calcium barium Fr francium Ra2 radium B boron C carbon nitride N3-oxide O2-fluoride F-neon Ne Al3 aluminum Si silicon phosphide P3-sulfide S2-chloride Cl-argon Ar helium He Zn2 zinc In3 indium Ge4 germanium As3-arsenide selenide Se2-bromide Br-krypton Kr telluride Te2-Po2 poloniumII poloniumIV Po4 iodide I-xenon Xe. Dissolve 68 g of potassium dihydrogen orthophosphate in sufficient water to produce l000ml. Fruits that can benefit from the addition of potassium dihydrogen phosphate includes common fruits peppers and roses. Polyatomic ions are ions which consist of more than one atom.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title potassium dihydrogen phosphate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.