Phosphoric acid properties
Home » chemical » Phosphoric acid properties >Phosphoric acid properties
Phosphoric Acid Properties. Your own stomach utilizes hydrochloric acid to digest food. H 2 SO 4 Also known as. Carbonated sodas contain phosphoric acid. Vinegar contains acetic acid.
 Chemistry In Dr Pepper Screen 11 On Flowvella Presentation Software For Mac Ipad And Iphone From flowvella.com
Chemistry In Dr Pepper Screen 11 On Flowvella Presentation Software For Mac Ipad And Iphone From flowvella.com
Citrus fruits such as oranges and lemons contain citric acid and ascorbic acid which is better known as vitamin C. Although normally clear to slightly yellow it may be dyed dark brown to alert. A phosphoric acid in the general sense is a phosphorus oxoacid in which each phosphorus atom is in the oxidation state 5 and is bonded to four oxygen atoms one of them through a double bond arranged as the corners of a tetrahedronTwo or more of these PO 4 tetrahedra may be connected by shared single-bonded oxygens forming linear or branched chains cycles or more complex structures. NON-VOLATILE ACIDS Sulfuric Acid and Phosphoric Acid 7908 Formulae. Vinegar contains acetic acid. When dissolved mineral.
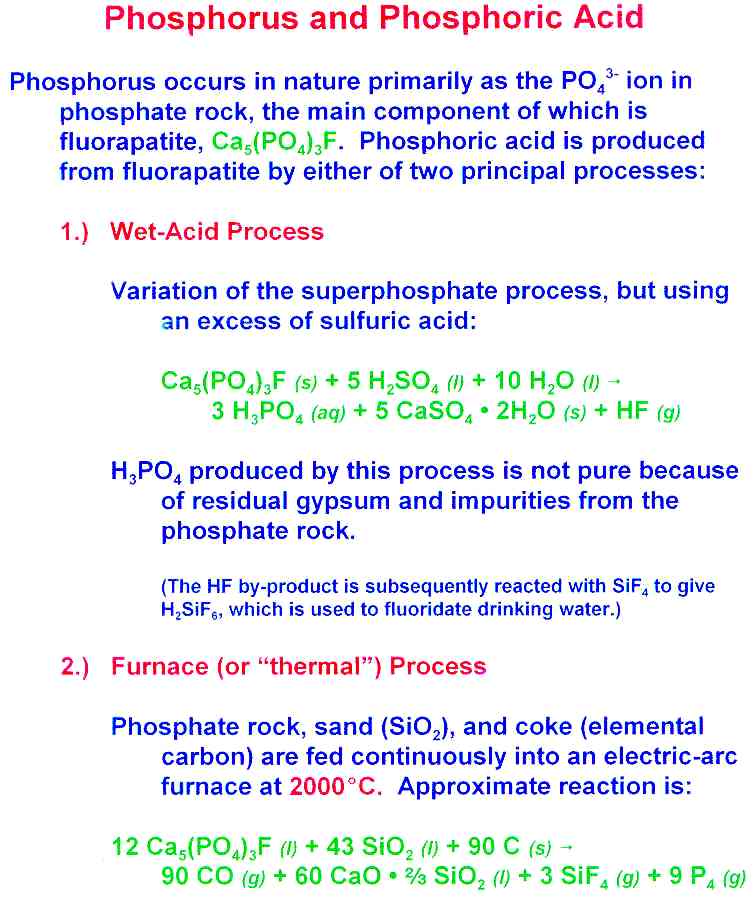
Uses of Phosphoric Acid Orthophosphoric acid is one of the important chemicals which has myriad of uses in several industries agriculture and products that we use in our daily lives.
The properties of a phospholipid are characterized by the. Acid of higher purity can also be made by using white phosphorus. The complex amino alcohols include choline ethanolamine and the amino acid-serine. On being heated together ester linkages are formed and water is a by-product. Glucose 6-phosphate C6H13O9P CID 5958 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. 21C 698F Page 5 of 8 MSDS -Phosphoric acid 85 Critical Temperature.
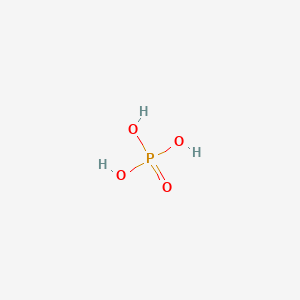
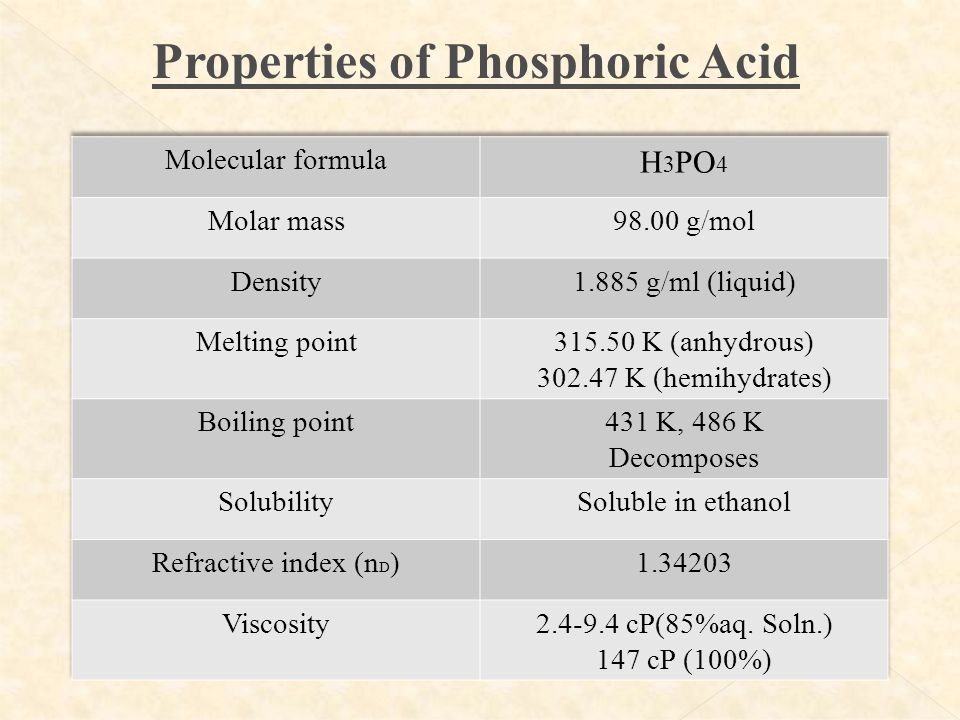
WS5600000 H 3 PO 4 MW. Your own stomach utilizes hydrochloric acid to digest food. Phosphoric acid also known as orthophosphoric acid or phosphoricV acid is a weak acid with the chemical formula H 3 P O 4The pure compound is a colorless solid. Phosphoric acid is also an important acid in biochemistry. NON-VOLATILE ACIDS Sulfuric Acid and Phosphoric Acid 7908 Formulae.
Source: chemspider.com
Phosphoric acid is also an important acid in biochemistry. The third oxygen on glycerol is bonded to phosphoric acid through a phosphate ester bond oxygen-phosphorus double bond oxygen. S25470B Recommended uses of the product and uses restrictions on use. May generate flammable andor toxic gases in contact with. Identification of the substancemixture and of the supplier Product name.
 Source: slideplayer.com
Source: slideplayer.com
The raw materials from which this high performance technical grade ceramic is made are readily available and reasonably priced resulting in good value for the cost in fabricated alumina shapes. Carbonated sodas contain phosphoric acid. Compound lipids a Phospholipids which yield fatty acids glycerol amino alcohol sphingosine phosphoric acid and nitrogen-containing alcohol upon hydrolysis. H 2 SO 4 Also known as. For the equilibrium dissociation reaction.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Vinegar contains acetic acid. Polyhedron publishes original fundamental experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. Once again you are unlikely ever to react this oxide with a base but you may well be expected to know how phosphoricV acid reacts with something like sodium hydroxide solution. Phosphoric AcidACS Created by Global Safety Management Inc. Can initiate the polymerization of certain classes of organic compounds.
 Source: flowvella.com
Source: flowvella.com
Acid of higher purity can also be made by using white phosphorus. Citrus fruits such as oranges and lemons contain citric acid and ascorbic acid which is better known as vitamin C. Phosphoric acid is referred to as being tribasic in. This is the chemical structure of sulfuric acid. Can initiate the polymerization of certain classes of organic compounds.
 Source: sciencestruck.com
Source: sciencestruck.com
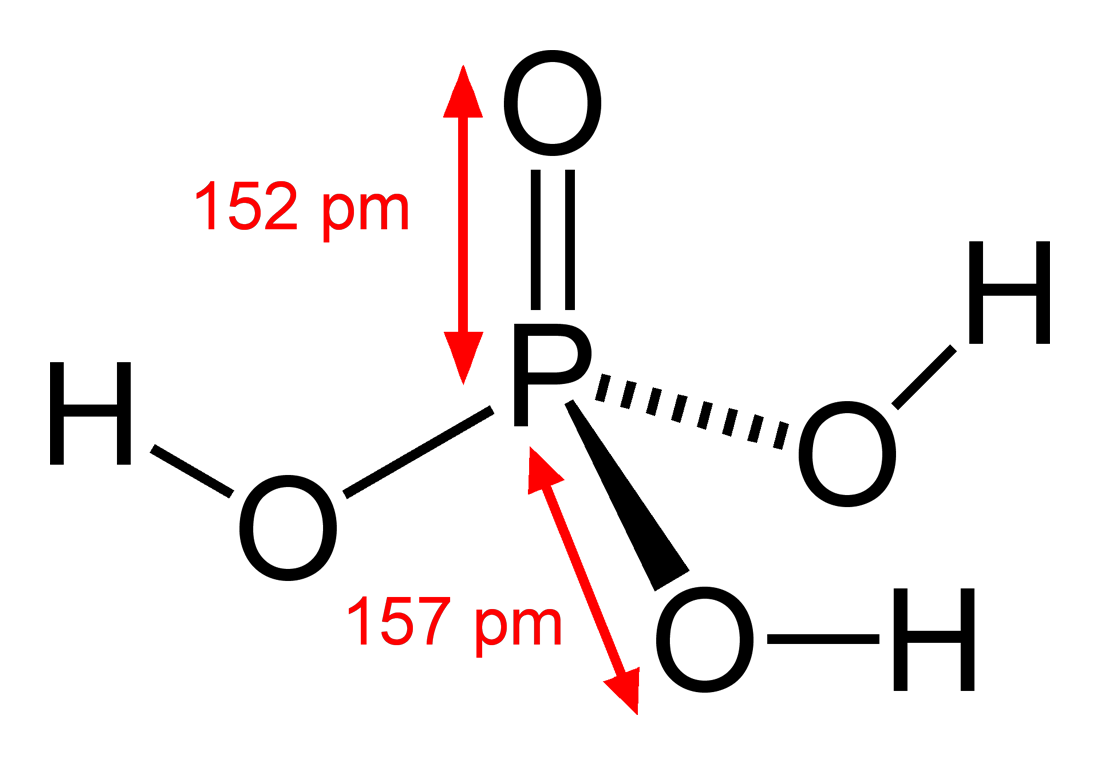
Although normally clear to slightly yellow it may be dyed dark brown to alert. All three hydrogens are acidic to varying degrees and can be lost from the molecule as H ions When all three H ions are removed the result is an orthophosphate ion PO 4 3 commonly called phosphate. A phosphoric acid in the general sense is a phosphorus oxoacid in which each phosphorus atom is in the oxidation state 5 and is bonded to four oxygen atoms one of them through a double bond arranged as the corners of a tetrahedronTwo or more of these PO 4 tetrahedra may be connected by shared single-bonded oxygens forming linear or branched chains cycles or more complex structures. 158C 3164F Melting Point. Properties of Aspirin acetylsalicylic acid Acidity Aspirin is a monoprotic weak acid K a 28 x 10-4 at 25 o C so very little of the molecular aspirin acetylsalicylic acid dissociates to form acetylsalicylate ions.
 Source: chem.tamu.edu
Source: chem.tamu.edu
Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Carbonated sodas contain phosphoric acid. Acids are a distinct class of compounds because of the properties of their aqueous solutions. The acidity of phosphoric acid may be reduced readily by natural water hardness minerals but the phosphate may persist indefinitely. PhosphoricV acid is also a weak acid with a pK a of 215.
 Source: slideplayer.com
Source: slideplayer.com
The complex amino alcohols include choline ethanolamine and the amino acid-serine. Alumina is one of the most cost effective and widely used material in the family of engineering ceramics. This includes synthetic chemistry coordination chemistry organometallic chemistry bioinorganic chemistry and solid-state and materials chemistry. Phosphoric acid is a mineral acid. Acid of higher purity can also be made by using white phosphorus.
 Source: researchgate.net
Source: researchgate.net
With an excellent combination of properties and an. Vinegar contains acetic acid. Acid any substance that in water solution tastes sour changes the color of certain indicators eg reddens blue litmus paper reacts with some metals eg iron to liberate hydrogen reacts with bases to form salts and promotes certain chemical reactions acid catalysis. Phosphoric acid also known as orthophosphoric acid or phosphoricV acid is a weak acid with the chemical formula H 3 P O 4The pure compound is a colorless solid. The complex amino alcohols include choline ethanolamine and the amino acid-serine.
 Source: simple.wikipedia.org
Source: simple.wikipedia.org
7908 Issue 1 EVALUATION. H 2 SO 4 Also known as. WS5600000 H 3 PO 4 MW. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. That makes it fractionally weaker than phosphorous acid.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title phosphoric acid properties by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.