Phosphate or phosphide
Home » chemical » Phosphate or phosphide >Phosphate or phosphide
Phosphate Or Phosphide. 2 Calcium phosphide SrI 2 Strontium iodide FeCl 2 IronII chloride or ferrous chloride 2. This anion is furthermore capable of sulfur-element bond formation through treatment with suitable electrophiles. An important example of an ester of a polyphosphate is ATP which is the monoester of triphosphoric acid H 5 P 3 O 10. Calcium nitride Ca 3 N 2.
 Are Phosphide And Phosphate The Same From moviecultists.com
Are Phosphide And Phosphate The Same From moviecultists.com
Always look for the presence of toxic preservative compounds in non-hazardous products such as. 2 copperII NO 3-nitrate Cu copperI SO 3 2-sulfite Cr. Al 4 C 3 aluminum carbide. 2 Calcium phosphide SrI 2 Strontium iodide FeCl 2 IronII chloride or ferrous chloride 2. NaC 2 H 3 O 2 - Sodium Acetate. 2-hydrogen phosphate H2PO4-dihydrogen phosphate Monatomic Cations 1 H hydrogen Li lithium Na sodium K potassium Rb rubidium Cs cesium Ag silver 2 Mg2 magnesium Ca2 calcium Sr2 strontium Ba2 barium Zn2 zinc Cd2 cadmium 3 Al3 aluminum Bi3 bismuth Monatomic Anions 1- H-hydride F-fluoride Cl chloride Br-bromide I-iodide 2- O2-oxide S2-sulfide 3- N3-nitride P3-phosphide 4-.
An ionic compound is made up of one or more anions and one or more cations.
Compounds containing polyatomic ions are named similarly to those containing only monatomic ions except there is no need to change to an ide ending since the suffix is already present in the name of the anion. Cs 3 N - Cesium Nitride. K 3 N - Potassium Nitride. Non-hazardous salts such as Sodium chloride Magnesium sulfate Potassium phosphate Calcium acetate etc. For example DNA and RNA are polymers of the type PO 2 OROR n. Nickel III phosphate 27 Li 2 SO 3 lithium sulfite 28 Zn 3 P 2 zinc phosphide 29 SrC 2 H 3 O 2 2 strontium acetate 30 Cu 2 O copper I oxide 31 Ag 3 PO 4 silver phosphate 32 YClO 3 yttrium I chlorate 33 SnS 2 tin IV sulfide 34 TiCN 4 titanium IV cyanide 35 KMnO 4 potassium permanganate 36 Pb 3 N 2 lead II nitride 37.

Aluminum sulfide Al 2 S 3. Li3P lithium phosphide 3. In the case of. 3 ironIII NH 4 ammonium Fe. 1 NaBr sodium bromide 2 ScOH3 scandium hydroxide 3 V2SO43 vanadium III sulfate 4 NH4F ammonium fluoride 5 CaCO3 calcium carbonate 6 NiPO4 nickel III phosphate 7 Li2SO3 lithium sulfite 8 Zn3P2 zinc phosphide 9 SrC2H3O22 trontium acetates.
 Source: pnas.org
Source: pnas.org
Beryllium chloride BeCl 2. An ionic compound is made up of one or more anions and one or more cations. 15 titanium IV nitrate TiNO34. ManganeseII 621 Write the name for each of the following ionic compounds. Boron iodide BI 3.

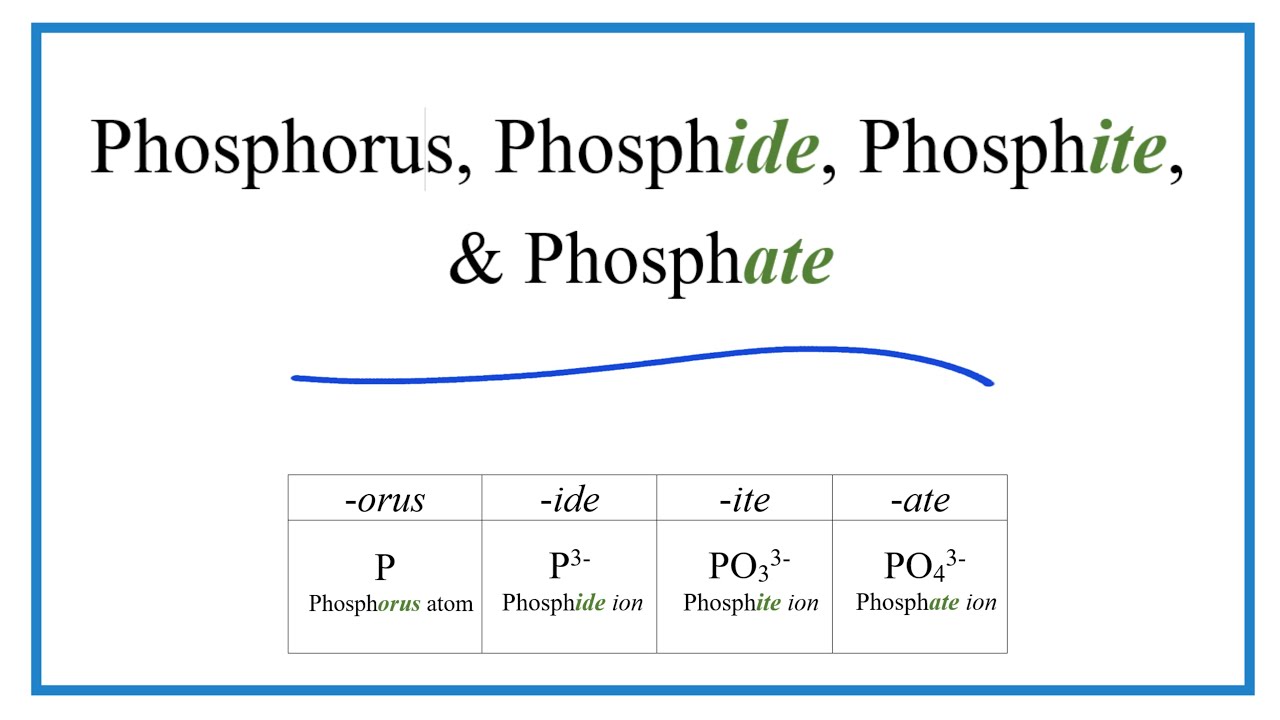
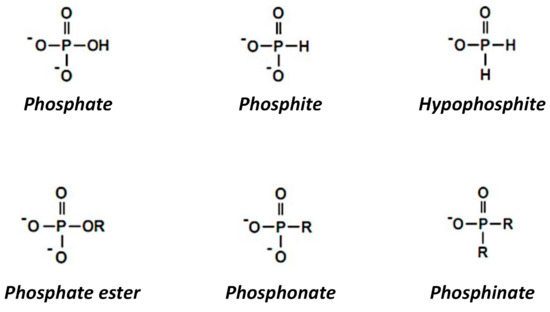
3 phosphate ClO 4 perchlorate HPO 4. Phosphate PO 4 3-phosphite PO 3 3-phosphide P3-Name. Sign up and fill out a short form to start offering your service. 17 copper I phosphate Cu3PO4. Cs 3 N - Cesium Nitride.
 Source: askdifference.com
Source: askdifference.com
3 phosphate ClO 4 perchlorate HPO 4. Trichlorosilane Phosphorous pentasulfide. Phosphate Compounds Powder 2 Phosphide Compounds Powder 1 Pigments Inorganic 1 Polymeric Powders 3 Rare Earth Materials RE REM REO Powder 2 Sand Silicon Dioxide Si02 3 Selenide Compounds Powder 2 Silicate Compounds Powder 7 Sol Gel Materials 1 Sputtering Target Materials CVD PVD 1 SRM Standard Reference Materials NIST ASTM 4 Sulfate Sulphate. Non-hazardous salts such as Sodium chloride Magnesium sulfate Potassium phosphate Calcium acetate etc. Cs 3 N - Cesium Nitride.
 Source: mdpi.com
Source: mdpi.com
Cs 3 N - Cesium Nitride. NaC 2 H 3 O 2 - Sodium Acetate. 3 ironIII NH 4 ammonium Fe. Boron iodide BI 3. K 3 N - Potassium Nitride.
 Source: sciencedirect.com
Source: sciencedirect.com
Write names for the following compounds and classify as ionic I or covalent C. 16 diselenium diiodide Se2I2. 2 copperII NO 3-nitrate Cu copperI SO 3 2-sulfite Cr. Zinc phosphate is an inorganic compound with the formula Zn 3 PO 4 2This white powder is widely used as a corrosion resistant coating on metal surfaces either as part of an electroplating process or applied as a primer pigment see also red leadIt has largely displaced toxic materials based on lead or chromium and by 2006 it had become the most commonly used corrosion inhibitor. Furthermore trichlorosilane reacts with sulfate generating trichlorosilyl sulfide isolated as its tetrabutylammonium salt.
 Source: sciencedirect.com
Source: sciencedirect.com
Cs 3 P - Cesium Phosphide. 13 iron II phosphide Fe3P2. LiClO 3 - Lithium Chlorate. Common Polyatomic Negative Ions-1 ions. Zinc phosphate is an inorganic compound with the formula Zn 3 PO 4 2This white powder is widely used as a corrosion resistant coating on metal surfaces either as part of an electroplating process or applied as a primer pigment see also red leadIt has largely displaced toxic materials based on lead or chromium and by 2006 it had become the most commonly used corrosion inhibitor.
 Source: moviecultists.com
Source: moviecultists.com
Phosphate pyrophosphate sulfate sulfite thiocyanate thiosulfate ammonium hydronium C2O4 2 ClO4 IO4 MnO4 O2 2 PO4 3 P2O7 4 SO4 2 SO3 2 SCN S2O3 2 NH4 H3O POSITIVE POLYATOMIC IONS TABLE OF POLYATOMIC IONS H2PO4 HCO3 HC2O4 HSO4 HS HSO3 OH ClO IO3 HPO4 2 NO3 NO2. Comments Optical and electron-energy-loss data for evaporated-aluminum films have been critically analyzed and used in an iterative self-consistent algorithm that represents a combination of the Kramers-Kronig analysis and the semiquantum-model application. Metals combine with polyatomic ions to give ionic compounds. Calcium nitride Ca 3 N 2. Sign up and fill out a short form to start offering your service.
 Source: askdifference.com
Source: askdifference.com
Compounds Containing Polyatomic Ions. Name the cation first specifying the charge if necessary then the polyatomic ion as listed in the table. 17 copper I phosphate Cu3PO4. 18 gallium oxide Ga2O3. HSO 4-hydrogen sulfate bisulfate CH 3 CO 2-acetate.
 Source: pnas.org
Source: pnas.org
Write names for the following compounds and classify as ionic I or covalent C. Polyphosphates also form esters. 2-hydrogen phosphate H2PO4-dihydrogen phosphate Monatomic Cations 1 H hydrogen Li lithium Na sodium K potassium Rb rubidium Cs cesium Ag silver 2 Mg2 magnesium Ca2 calcium Sr2 strontium Ba2 barium Zn2 zinc Cd2 cadmium 3 Al3 aluminum Bi3 bismuth Monatomic Anions 1- H-hydride F-fluoride Cl chloride Br-bromide I-iodide 2- O2-oxide S2-sulfide 3- N3-nitride P3-phosphide 4-. Do NOT use prefixes to. Furthermore trichlorosilane reacts with sulfate generating trichlorosilyl sulfide isolated as its tetrabutylammonium salt.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title phosphate or phosphide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.