Perchloric acid cas
Home » chemical » Perchloric acid cas >Perchloric acid cas
Perchloric Acid Cas. Bismuth has been used in solders a variety of other alloys metallurgical additives and medications and in atomic research. Colorless when freshly made but rapidly turns yellowish or brown on exposure to light and air. The azeotrope 569 HI boils at 127 C. This process is the main outlet for the industrial production of perchloric acid.
 Perchloric Acid 70 Percent Reagent Acs Cas 7601 90 3 P1025 Spectrum Chemical From spectrumchemical.com
Perchloric Acid 70 Percent Reagent Acs Cas 7601 90 3 P1025 Spectrum Chemical From spectrumchemical.com
Name CAS by Weight Sulfuric Acid 7664-93-9 93 Toxicological Data on Ingredients. It will contract slightly on freezing. 7300 Issue 3 EVALUATION. Page 3 of 8 Section 3. Ammonium perchlorate AP is produced by reaction between ammonia and perchloric acid. 15 August 1990 Issue 3.
CAS Number Choose CAS Number 100-82-7 108-88-3 109-89-7 109-99-9 110-82-7 110-87-7 111-30-8 1239-45-8 1310-73-2 1336-21-6 141-78-6 142-82-5 50-00-0 64-17-5 64-19-7 64-68-5 67-56-1 67-63-0 67-64-1 67-66-3 67-68-5 68-12-2 75-05-8 75-09-2 75-15-0 7601-90-3 7647-01-0 7664-39-3 7664-93-9 7681-52-9 7697-37-2 77-92-9 7722-84-1.
15 March 2003 OSHA. The salt also can be produced by salt metathesis reaction of ammonium salts with sodium perchlorate. 510 mgm 2 hours Rat. Reacts violently with acetic anhydride 2-aminoethanol ammonium hydroxide calcium phosphide chlorosulfonic acid 11-difluoroethylene ethylenediamine ethyleneimine oleum perchloric acid b-propiolactone propylene oxide silver perchloratecarbon tetrachloride mixture sodium hydroxide uraniumIV phosphide vinyl acetate calcium carbide rubidium carbide cesium acetylide rubidium. CAS Number Choose CAS Number 100-82-7 108-88-3 109-89-7 109-99-9 110-82-7 110-87-7 111-30-8 1239-45-8 1310-73-2 1336-21-6 141-78-6 142-82-5 50-00-0 64-17-5 64-19-7 64-68-5 67-56-1 67-63-0 67-64-1 67-66-3 67-68-5 68-12-2 75-05-8 75-09-2 75-15-0 7601-90-3 7647-01-0 7664-39-3 7664-93-9 7681-52-9 7697-37-2 77-92-9 7722-84-1. Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower.
 Source: chemicals.co.uk
Source: chemicals.co.uk
Empirical Formula Hill Notation. C 26 H 42 N 7 O 19 P 3 S. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Section 8 - Exposure Controls Personal Protection Engineering Controls. SbNO 2 3 antimony III nitrite HIO hypoiodous acid NH 4 2CO 3 ammonium carbonate LiC ℓO 4 lithium perchlorate HC ℓO 2 chlorous acid HCH 3COO acetic acid Au 3PO 3 gold I phosphite Cu 3BO 3 copper I borate HNO 2 nitrous acid H 3PO 3 phosphor ous acid.
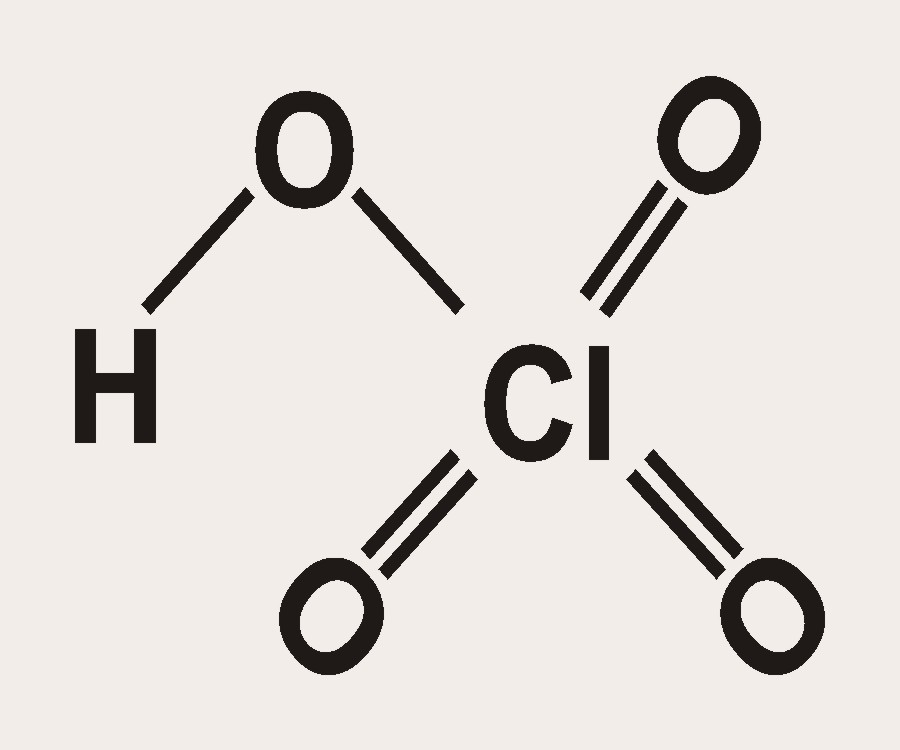 Source: seastarchemicals.com
Source: seastarchemicals.com
320 mgm 2 hours Mouse. Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. 510 mgm 2 hours Rat. 15 August 1990 Issue 3. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
 Source: en.wikipedia.org
Source: en.wikipedia.org
C 26 H 42 N 7 O 19 P 3 S. Section 8 - Exposure Controls Personal Protection Engineering Controls. The salt also can be produced by salt metathesis reaction of ammonium salts with sodium perchlorate. Very hazardous in case of skin contact corrosive irritant permeator of eye contact. 510 mgm 2 hours Rat.
 Source: chemsrc.com
Source: chemsrc.com
Name CAS by Weight Sulfuric Acid 7664-93-9 93 Toxicological Data on Ingredients. High Purity Lab Chemicals And High Quality Lab Supplies For Sale Online In The USA. Colorless when freshly made but rapidly turns yellowish or brown on exposure to light and air. It has a metallic luster and is silver-white with an iridescent tarnishAmong the heavy metals it is the heaviest and the only non-toxic. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
 Source: actu-all.com
Source: actu-all.com
Aluminum calcium lanthanum nickel strontium tungsten antimony chromium lithium potassium tellurium vanad ium arsenic cobalt magnesium. Very hazardous in case of skin contact corrosive irritant permeator of eye contact. 15 August 1990 Issue 3. Page 3 of 8 Section 3. NitricPerchloric Acid Ashing MW.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It will contract slightly on freezing. 15 August 1990 Issue 3. SbNO 2 3 antimony III nitrite HIO hypoiodous acid NH 4 2CO 3 ammonium carbonate LiC ℓO 4 lithium perchlorate HC ℓO 2 chlorous acid HCH 3COO acetic acid Au 3PO 3 gold I phosphite Cu 3BO 3 copper I borate HNO 2 nitrous acid H 3PO 3 phosphor ous acid. Name CAS by Weight Sulfuric Acid 7664-93-9 93 Toxicological Data on Ingredients. Bismuth is mainly a byproduct of lead ore processing.
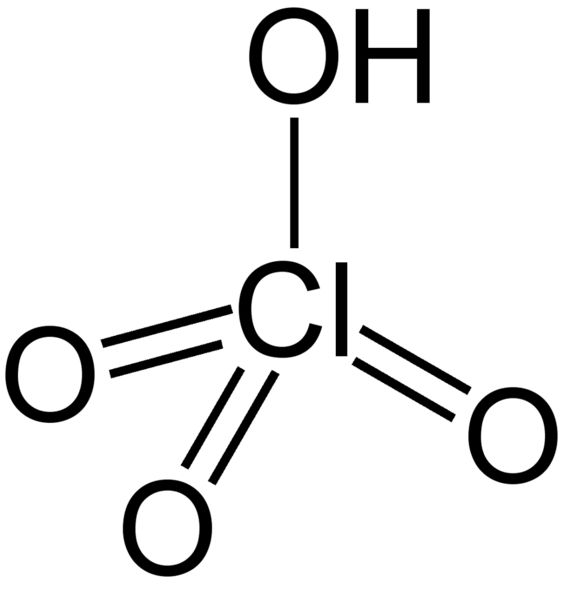 Source: sielc.com
Source: sielc.com
Compare Product No. 320 mgm 2 hours Mouse. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Acids and Review 1. Bismuth is mainly a byproduct of lead ore processing.
 Source: spectrumchemical.com
Source: spectrumchemical.com
Empirical Formula Hill Notation. In the early 1990s research began on the evaluation of bismuth as a nontoxic. SbNO 2 3 antimony III nitrite HIO hypoiodous acid NH 4 2CO 3 ammonium carbonate LiC ℓO 4 lithium perchlorate HC ℓO 2 chlorous acid HCH 3COO acetic acid Au 3PO 3 gold I phosphite Cu 3BO 3 copper I borate HNO 2 nitrous acid H 3PO 3 phosphor ous acid. It will contract slightly on freezing. Reacts violently with acetic anhydride 2-aminoethanol ammonium hydroxide calcium phosphide chlorosulfonic acid 11-difluoroethylene ethylenediamine ethyleneimine oleum perchloric acid b-propiolactone propylene oxide silver perchloratecarbon tetrachloride mixture sodium hydroxide uraniumIV phosphide vinyl acetate calcium carbide rubidium carbide cesium acetylide rubidium.
 Source: m.fischerchem.com
Source: m.fischerchem.com
C 26 H 42 N 7 O 19 P 3 S. Miscible with water and alcohol. Very hazardous in case of skin contact corrosive irritant permeator of eye contact. If they begin with hydrogen name them as acids. Chloric acid H Cl O 3 is an oxoacid of chlorine and the formal precursor of chlorate salts.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Hazards Identification Potential Acute Health Effects. The salt also can be produced by salt metathesis reaction of ammonium salts with sodium perchlorate. Page 2 of 8. Very hazardous in case of skin contact corrosive irritant permeator of eye contact. It has a metallic luster and is silver-white with an iridescent tarnishAmong the heavy metals it is the heaviest and the only non-toxic.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title perchloric acid cas by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.