P2o5 react with water
Home » chemical » P2o5 react with water >P2o5 react with water
P2o5 React With Water. Cover with DRY earth DRY sand or other non-combustible material followed with plastic sheet to minimize spreading or contact with rain. Thermodynamic properties of substances The solubility of the substances Periodic table of elements. This method is not applicable to plastic samples containing volatile compounds. Consider the following reaction between sulfur trioxide and water.
 Mechanism Of Carboxylic Acid And Amide Dehydration With Phosphorus Pentoxide Chemistry Stack Exchange From chemistry.stackexchange.com
Mechanism Of Carboxylic Acid And Amide Dehydration With Phosphorus Pentoxide Chemistry Stack Exchange From chemistry.stackexchange.com
Therefore MAP fertilizer granules are acidic and it makes this fertilizer very useful. NH4 If the difference is between. NOx and volatile organic compounds react in the at mosphere. C 2 H 6 O 2 O 2 CO 2 H 2 O Chemical equations must be mass balanced and. Neutralization reaction occurs between Acetic acid CH_3COOHAcid Ammonia NH_3Base to produce Ammonium acetate CH_3COO-NH_4Salt CH_. Knowing these differences helps us to understand why zinc which does not react with water is used in roofing nails roof flashings and rain gutters and sodium is not.
Assertion Reason Solid State QNo1 Which of the following is not a characteristic property of Solids.
That is they can react as acids. 01 g and record the mass in your data table. While the compound P4O10 has many names its most common name is phosphorus pentoxide. Sulfuric acid aq zinc hydroxide s arrow zinc sulfate aq water l a. In our study of chemistry we will see why substances differ in properties and how these. The technical method production phosphoric acid.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
And the saturation level of sorption complex with alkaline cations. You can obtain your crystals while your crucible is cooling. And the saturation level of sorption complex with alkaline cations. Also we can appreciate why gold because of its chemical inertness is prized for jewelry and coins. The combustion of these compounds generates mixed oxides of nitrogen NOx.
 Source: youtube.com
Source: youtube.com
SO3gH2OlH2SO4aq A chemist allows 615 g of SO3 and 112 g of H2O to react. Other ionic compounds may have different structures and different charges as long as the crystal remains. P4O10 P2O5 6 H2O 4 H3PO4. When dissolving in soil water the two components in the fertilizer separate from each other releasing ammonium ions and phosphate ions. Metals react with nonmetals to form ionic compounds salts.

Consider the following reaction between sulfur trioxide and water. Keep combustibles wood paper oil etc away from spilled material. Use clean non-sparking tools to collect material and place it into. Also we can appreciate why gold because of its chemical inertness is prized for jewelry and coins. 2 P2O5 covalent compound diphosphorus pentoxide or more usually phosphorus V oxide.
 Source: sciencedirect.com
Source: sciencedirect.com
The Hazard fields include special hazard alerts air and water reactions fire hazards. _ 18 g _ 09 g o 735 g _ 368 g. NH4 If the difference is between. Answer 1 of 6. It is a water-soluble substance and it can rapidly dissolve in moist soil.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
After the sample has been decomposed and. In our study of chemistry we will see why substances differ in properties and how these. 168 g of calcium nitrate and 1750 g of ammonium fluoride react completely to form calcium fluoride dinitrogen monoxide and water vapor. Therefore MAP fertilizer granules are acidic and it makes this fertilizer very useful. Cs3n ionic or covalent.
 Source: slideplayer.com
Source: slideplayer.com
Ionic Compounds When an element composed of atoms that readily lose electrons a metal reacts with an element composed of atoms that readily gain electrons a nonmetal. NH4 If the difference is between. Therefore MAP fertilizer granules are acidic and it makes this fertilizer very useful. Sulfuric acid aq zinc hydroxide s arrow zinc sulfate aq water l a. In our study of chemistry we will see why substances differ in properties and how these.
 Source: youtube.com
Source: youtube.com
Knowing these differences helps us to understand why zinc which does not react with water is used in roofing nails roof flashings and rain gutters and sodium is not. Determine the limiting reactant for the reaction. The calculated mass of water lost from the compound is 0. Lets try with ethylene glycol combustion. The percentage of P2O5 present in the sample is A.
 Source: youtube.com
Source: youtube.com
Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. 168 g of calcium nitrate and 1750 g of ammonium fluoride react completely to form calcium fluoride dinitrogen monoxide and water vapor. 21092014 Evaluation of information. Also notice that the coefficient for butane 2 is one-fourth the coefficient of carbon dioxide 8. This method is not applicable to plastic samples containing volatile compounds.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Ionic Compounds When an element composed of atoms that readily lose electrons a metal reacts with an element composed of atoms that readily gain electrons a nonmetal. When the reaction is finished the chemist collects 511 g of H2SO4. Use water spray to reduce vapors. Notice that the coefficient for water 10 is five times that of butane 2. Concentrations of available components such as P2O5 K2O and Mg.
 Source: slideplayer.com
Source: slideplayer.com
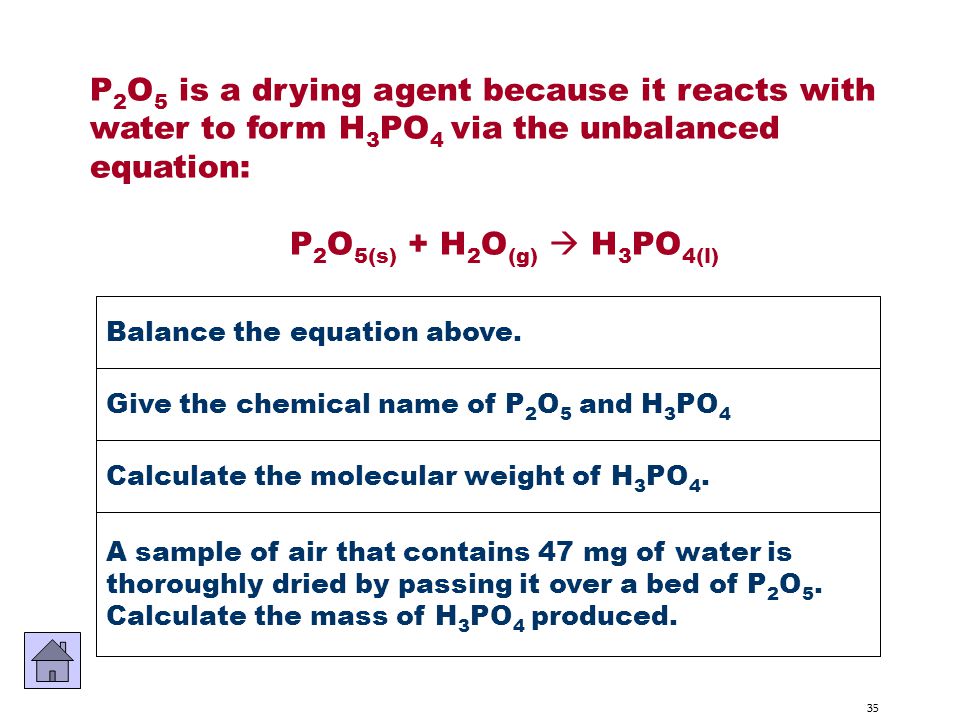
P4O10 P2O5 6 H2O 4 H3PO4. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. The Hazard fields include special hazard alerts air and water reactions fire hazards. Hypochlorite ions formed by the chlorination of water for disinfection purposes may react with acetone to form chloroform. When the reaction is finished the chemist collects 511 g of H2SO4.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title p2o5 react with water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.