Molecular mass of nitrogen monoxide
Home » chemical » Molecular mass of nitrogen monoxide >Molecular mass of nitrogen monoxide
Molecular Mass Of Nitrogen Monoxide. The mole is an important concept for talking about a very large number of things 602 x 10 23 of them to be exact. They are used in the petroleum industry for the type separation of naphthenes. 185 Occurrence Preparation and Compounds of Hydrogen. 3D left and center and 2D right representations of the terpenoid molecule atisane.
 Day 33 Notes Chapter 7 Chemical Quantities The Mole Ppt Download From slideplayer.com
Day 33 Notes Chapter 7 Chemical Quantities The Mole Ppt Download From slideplayer.com
Examples are glucose CH 2 O. As a result of heterogeneous population of the activated precursor ions a series of peptide bonds are cleaved 161. Molecular weightmolar mass of NO 2. The human body contains about 3 nitrogen by mass the fourth most abundant element in the body after oxygen carbon. The molar fraction of each C 2 product excluding hydrogen at 200 mA cm 2 in each amine system is shown in Fig. The molar mass is simply the mass of one mole of substance ie.
Type 13X offers enhanced adsorption performance over the type A zeolite.
182 Occurrence and Preparation of the Representative Metals. Examples are glucose C 6 H 12 O 6. Molecular Weight of some Common Substances - Definition and molecular weight molar mass of some common substances. Molecular sieves of the 13X type are also used. For example CO is named carbon monoxide rather than carbon oxide. The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 112 of the mass of a neutral carbon-12 12 C isotope atom.
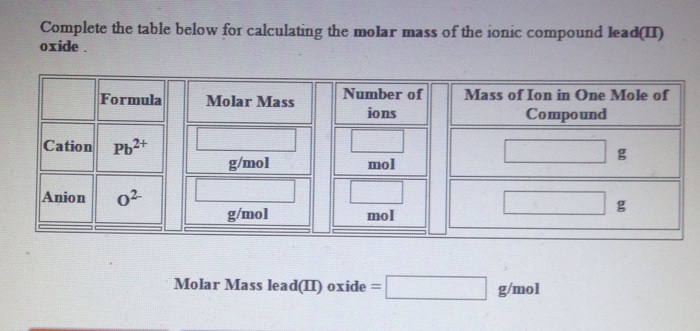
Combustion generated oxides of nitrogen are. It tells the actual number of atoms of an element in a compound. 184 Structure and General Properties of the Nonmetals. As a result of heterogeneous population of the activated precursor ions a series of peptide bonds are cleaved 161. Ethene C 2 H 4.
 Source: youtube.com
Source: youtube.com
It tells the actual number of atoms of an element in a compound. Carbon Monoxide The affinity of hemoglobin in the blood for carbon monoxide is 200300 times greater than its affinity for oxygen. It tells the actual number of atoms of an element in a compound. For example the molecular weight of water would be obtained by the following process. Since N 2 is isoelectronic with carbon monoxide CO and acetylene C 2 H 2 the bonding in dinitrogen complexes is closely allied to that in carbonyl compounds although N 2 is a weaker σ-donor and π-acceptor than CO.
 Source: slideplayer.com
Source: slideplayer.com
Writing the Formula From the Name. You can write the formula for a covalent compound. Natural Gas Consumption - Natural gas consumption for equipment like boiling pans ovens cookers kettles and more. Its molecular mass is 128 g mol-1 calculate its molecular formula. Type 13X offers enhanced adsorption performance over the type A zeolite.
 Source: youtube.com
Source: youtube.com
Writing the Formula From the Name. The molar mass is simply the mass of one mole of substance ie. For example the molecular weight of water would be obtained by the following process. 182 Occurrence and Preparation of the Representative Metals. Since both of these elements are diatomic in air - O 2 and N 2 the molar mass of oxygen gas is 32 gmol and the molar mass of nitrogen gas is 28 gmol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
For example the molecular weight of water would be obtained by the following process. Fuels such as nitrogen carbon dioxide carbon monoxide and other trace gases can also act as asphyxiants by dis-placing oxygen. It describes 19th-century developments that led to the concept of the mole Topics include atomic weight molecular weight and molar mass. Although there are no ions in these compounds they are named in a similar manner to binary ionic compounds. Since N 2 is isoelectronic with carbon monoxide CO and acetylene C 2 H 2 the bonding in dinitrogen complexes is closely allied to that in carbonyl compounds although N 2 is a weaker σ-donor and π-acceptor than CO.
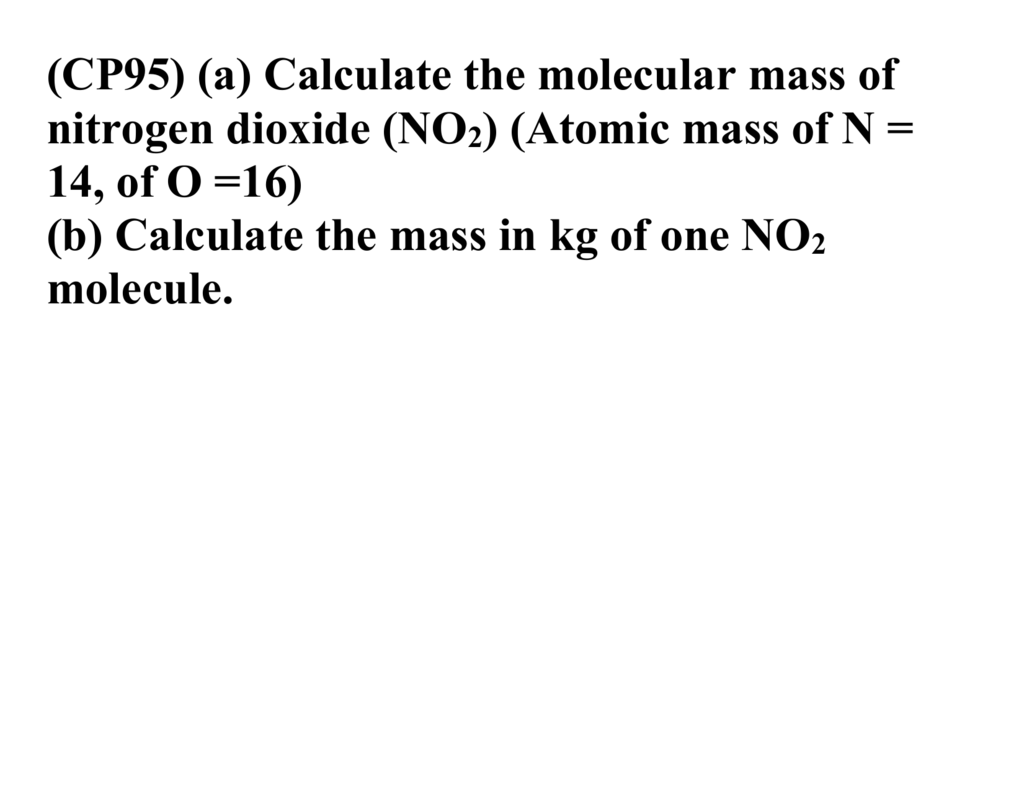 Source: studylib.net
Source: studylib.net
Writing the Formula From the Name. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. For example CO is named carbon monoxide rather than carbon oxide. 184 Structure and General Properties of the Nonmetals. The 13X molecular sieve is the sodium form of zeolite X and has a much larger pore opening than the type A crystals with an effective pore diameter of 10 angstroms.
 Source: youtube.com
Source: youtube.com
Nitrogen - Specific Heat - Specific heat of Nitrogen Gas - N 2 - at temperatures ranging 175 - 6000 K. Type 13X offers enhanced adsorption performance over the type A zeolite. As a result of heterogeneous population of the activated precursor ions a series of peptide bonds are cleaved 161. The human body contains about 3 nitrogen by mass the fourth most abundant element in the body after oxygen carbon. Density of Nitrogen dioxide.
 Source: slideplayer.com
Source: slideplayer.com
3dData for pure CO electrolysis. Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. For example CO is named carbon monoxide rather than carbon oxide. You can write the formula for a covalent compound.
 Source: kenyaplex.com
Source: kenyaplex.com
Nitrogen - Specific Heat - Specific heat of Nitrogen Gas - N 2 - at temperatures ranging 175 - 6000 K. Nitrogen - Specific Heat - Specific heat of Nitrogen Gas - N 2 - at temperatures ranging 175 - 6000 K. It tells the actual number of atoms of an element in a compound. Molecular mass of H 2 O 2 x atomic mass of H 1 x atomic mass of O 2 x 100797 1 x 159994 amu 1802 amu. Molecular sieves are synthetic and naturally.
 Source: youtube.com
Source: youtube.com
Now the question is how one can determine the molecular mass of the analyte with the help of the ion signals. Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol. Chemical compound - chemical compound - Binary molecular covalent compounds. Density of Nitrogen dioxide. Fuels such as nitrogen carbon dioxide carbon monoxide and other trace gases can also act as asphyxiants by dis-placing oxygen.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molecular mass of nitrogen monoxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.