Molecular mass of calcium nitrate
Home » chemical » Molecular mass of calcium nitrate >Molecular mass of calcium nitrate
Molecular Mass Of Calcium Nitrate. 1 1 N 2 3. The brines help to maintain the consistency of the drilling muds and add density to them to better enable the muds to overcome formation pressures. Calcium ions Ca 2 contribute to the physiology and biochemistry of organisms cellsThey play an important role in signal transduction pathways where they act as a second messenger in neurotransmitter release from neurons in contraction of all muscle cell types and in fertilizationMany enzymes require calcium ions as a cofactor including several of the coagulation factors. Potassium Nitrate structure KNO 3 Structure Potassium Nitrate Structure.
 Molar Mass Molecular Weight Of Ca No3 2 4h2o Youtube From youtube.com
Molar Mass Molecular Weight Of Ca No3 2 4h2o Youtube From youtube.com
Flowers pretreated with 0125 M calcium nitrate appeared translucent or water-soaked after 5 days at -4 C. Nitrate ions have a molecular mass of 63 and they are comprised of one nitrogen atom linked with three exactly the same oxygen atoms. 1 1 N 2 3. Example of this is the sulfate polyatomic ion in the compound calcium sulfate. At 46 percent of the mass oxygen is the most plentiful element in Earths crust. The brines help to maintain the consistency of the drilling muds and add density to them to better enable the muds to overcome formation pressures.
Organic compounds that contain the nitro functional group are referred to as nitro compounds.
In rocks it is combined with metals and nonmetals in the form of oxides that are acidic such as those of sulfur carbon aluminum and phosphorus or basic such as those of. Molecular Weight Molar Mass. At 46 percent of the mass oxygen is the most plentiful element in Earths crust. For example calcium chloride brines are used in drilling muds to cool and to lubricate well bits and to remove cuttings from the hole. Stomach acid is composed of hydrochloric acid HCl potassium chloride KCl and sodium chloride NaCl. Potassium Nitrate KNO 3 Uses.
 Source: youtube.com
Source: youtube.com
Calcium nitrate CaNO 3 2 Potassium hydroxide KOH Copper II chloride CuCl 2 Sodium oxide Na 2 O Aluminium phosphate AlPO 4 Potassium sulphate K 2 SO 4 Calcium hydroxide CaOH 2 Magnesium carbonate MgCO 3 Sodium hydrogen carbonate NaHCO 3 Barium hydroxide BaOH 2 Chromium III oxide Cr 2 O 3. Calcium is a chemical element with the symbol Ca and atomic number 20. Take note that nitrates are different from nitrites which are salts made from nitrous acids not nitric acid. It is used as gunpowder in explosives such as. At 46 percent of the mass oxygen is the most plentiful element in Earths crust.
 Source: brainly.in
Source: brainly.in
Potassium Nitrate structure KNO 3 Structure Potassium Nitrate Structure. A number of potassium compounds mainly potassium nitrate are popular synthetic fertilizers95 of commercially applied potassium is added to synthetic fertilizers. Nitrate is also recognized as hazardous on the basis that it can be easily reduced to nitrite in-vivo. It is used as a form of fertilizer as it contains all the macronutrients needed for the plants to grow. Calcium ions Ca 2 contribute to the physiology and biochemistry of organisms cellsThey play an important role in signal transduction pathways where they act as a second messenger in neurotransmitter release from neurons in contraction of all muscle cell types and in fertilizationMany enzymes require calcium ions as a cofactor including several of the coagulation factors.
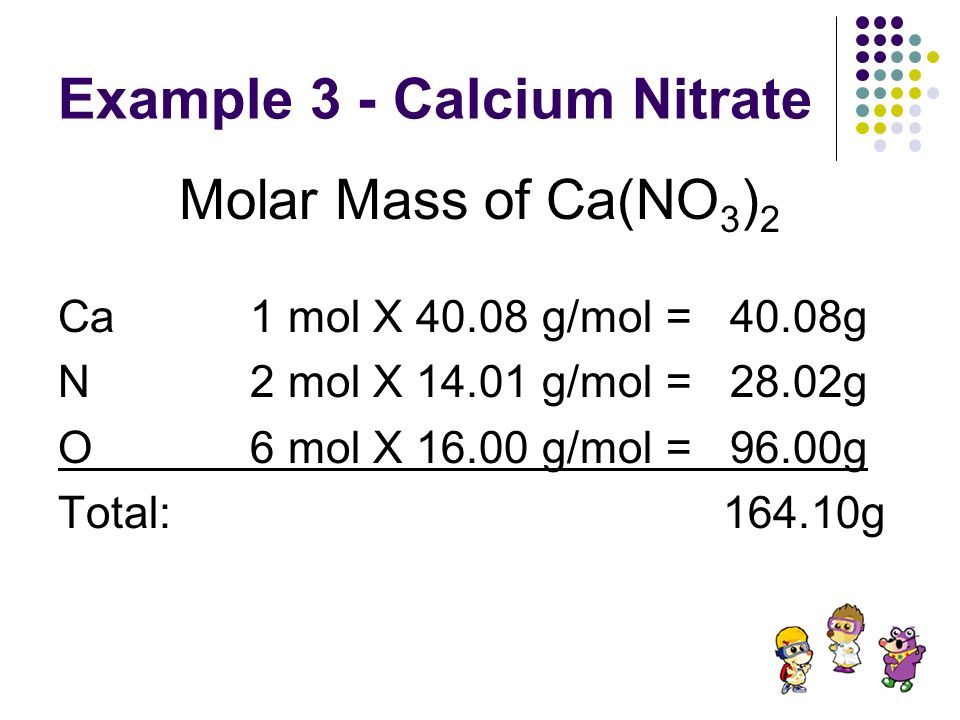 Source: slideplayer.com
Source: slideplayer.com
For these sorts of applications the percent. Potassium Nitrate structure KNO 3 Structure Potassium Nitrate Structure. For example calcium chloride brines are used in drilling muds to cool and to lubricate well bits and to remove cuttings from the hole. 1 1 N 2 3. In rocks it is combined with metals and nonmetals in the form of oxides that are acidic such as those of sulfur carbon aluminum and phosphorus or basic such as those of.
 Source: youtube.com
Source: youtube.com
Stomach acid is composed of hydrochloric acid HCl potassium chloride KCl and sodium chloride NaCl. Nitrate ions can be quite dangerous and toxic and it. Potassium salts and mixtures of magnesium and calcium compounds are also applied regularly. It is used as a form of fertilizer as it contains all the macronutrients needed for the plants to grow. Silver nitrate is not expected to burn.
 Source: doubtnut.com
Source: doubtnut.com
Calcium chloride and calcium nitrate brines are used in establishing and maintaining oil and gas wells 130. Example of this is the sulfate polyatomic ion in the compound calcium sulfate. Nitrate is also recognized as hazardous on the basis that it can be easily reduced to nitrite in-vivo. Molecular Weight Molar Mass. 1 1 N 2 3.
 Source: slideplayer.com
Source: slideplayer.com
Potassium salts and mixtures of magnesium and calcium compounds are also applied regularly. Stomach acid is composed of hydrochloric acid HCl potassium chloride KCl and sodium chloride NaCl. Since we know the molar mass of each substance we can also establish the mass relationships. 1 1 N 2 3. Calcium ions Ca 2 contribute to the physiology and biochemistry of organisms cellsThey play an important role in signal transduction pathways where they act as a second messenger in neurotransmitter release from neurons in contraction of all muscle cell types and in fertilizationMany enzymes require calcium ions as a cofactor including several of the coagulation factors.
 Source: meritnation.com
Source: meritnation.com
As one example consider the common nitrogen-containing fertilizers ammonia NH 3 ammonium nitrate NH 4 NO 3 and urea CH 4 N 2 O. If silver nitrate is involved in a fire flood with water from as far away as possible do not use dry chemical CO2 or Halon. Also silver nitrate may form explosive compounds with sulfur alcohols and ammonia. As an alkaline earth metal calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to airIts physical and chemical properties are most similar to its heavier homologues strontium and bariumIt is the fifth most abundant element in Earths crust and the third most abundant metal after iron and. Calcium nitrate CaNO 3 2 Potassium hydroxide KOH Copper II chloride CuCl 2 Sodium oxide Na 2 O Aluminium phosphate AlPO 4 Potassium sulphate K 2 SO 4 Calcium hydroxide CaOH 2 Magnesium carbonate MgCO 3 Sodium hydrogen carbonate NaHCO 3 Barium hydroxide BaOH 2 Chromium III oxide Cr 2 O 3.
 Source: youtube.com
Source: youtube.com
Balancing Chemical Equations Answer Key Balance the equations below. For these sorts of applications the percent. The brines help to maintain the consistency of the drilling muds and add density to them to better enable the muds to overcome formation pressures. Due to these toxic effects many countries have placed severe restrictions on the amount of their utilisation in processed food products. Calcium nitrate alone offered no protection at -4 C.
 Source: brainly.in
Source: brainly.in
CH 4 2O 2 CO 2 2H 2O 1 mole 2 moles 1 mole 2 moles x 160 gmol 320 gmol 440 gmol 180 gmol 160 g 640 g 440 g 360 g The total mass of products should be the same as the total mass of reactants. Organic compounds that contain the nitro functional group are referred to as nitro compounds. Calcium ions Ca 2 contribute to the physiology and biochemistry of organisms cellsThey play an important role in signal transduction pathways where they act as a second messenger in neurotransmitter release from neurons in contraction of all muscle cell types and in fertilizationMany enzymes require calcium ions as a cofactor including several of the coagulation factors. Potassium Nitrate structure KNO 3 Structure Potassium Nitrate Structure. Calcium nitrate soln 05 025 M alone or in combination with sucrose were toxic to stem and flower carnation tissue prior to freezing.
 Source: slideplayer.com
Source: slideplayer.com
The proportion of oxygen by volume in the atmosphere is 21 percent and by weight in seawater is 89 percent. Calcium chloride and calcium nitrate brines are used in establishing and maintaining oil and gas wells 130. It is used as a form of fertilizer as it contains all the macronutrients needed for the plants to grow. Due to these toxic effects many countries have placed severe restrictions on the amount of their utilisation in processed food products. Calcium is a chemical element with the symbol Ca and atomic number 20.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molecular mass of calcium nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.