Molecular mass of ammonium nitrate
Home » chemical » Molecular mass of ammonium nitrate >Molecular mass of ammonium nitrate
Molecular Mass Of Ammonium Nitrate. This compound is also known as Ammonium Nitrate. HCl Acid Hydrochloric Acid - A mineral acid also known as muriatic acid is a poisonous corrosive hazardous liquid that reacts with most metals to form explosive hydrogen gas and causes severe burns and irritation of eyes and mucous membranes. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. Used to manufacture ammonium nitrate.
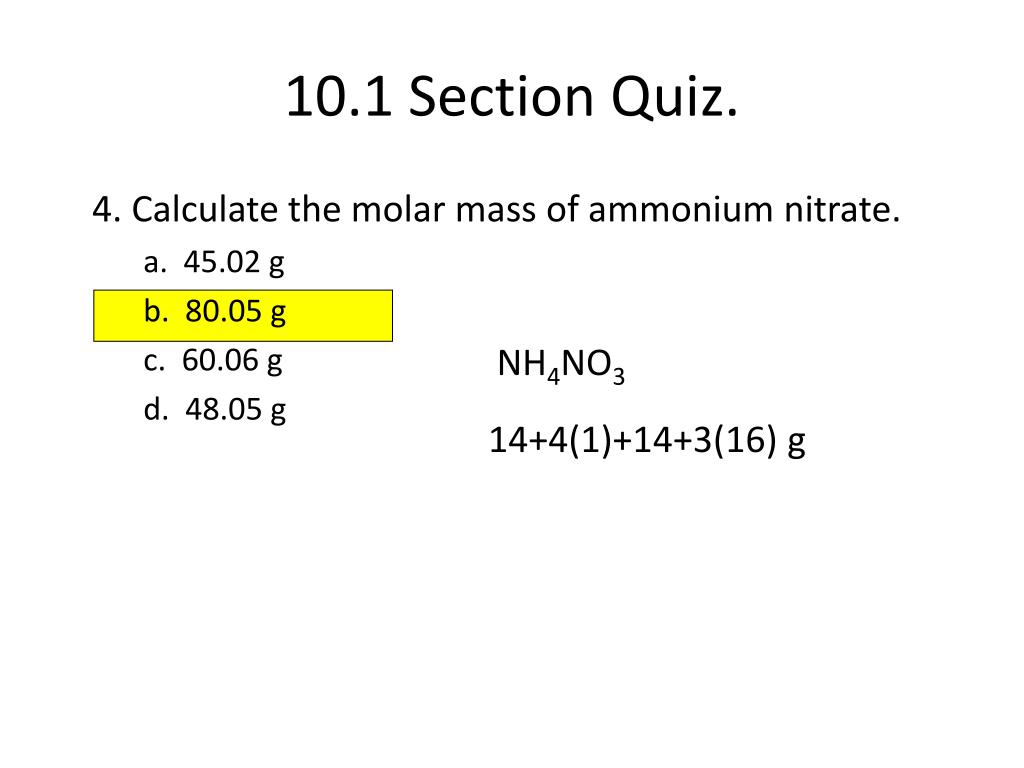
 Calculate The Percentage Of Nitrogen And Oxygen In Ammonium Nitrate Relative Molecular Mass Of Ammonium Nitrate Is 80 H 1 N 14 O 16 From wiredfaculty.com
Calculate The Percentage Of Nitrogen And Oxygen In Ammonium Nitrate Relative Molecular Mass Of Ammonium Nitrate Is 80 H 1 N 14 O 16 From wiredfaculty.com
The signals of NO mz 30 and NH 3 mz 17 were recorded indicating that NO is one of the intermediates in the NO 3-RR. Nitrate is a polyatomic ion with the chemical formula NO 3. Used in industries as a viscosity adjuster. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. For molecules with a. What is relative formula mass and relative molecular mass.
Almost all inorganic nitrates.
Silver nitrate is not expected to burn. Nitrate is a polyatomic ion with the chemical formula NO 3. Silver nitrate is not expected to burn. H 1 O 16 N 14 Solution. Organic compounds that contain the nitro functional group are referred to as nitro compounds. Used as a desensitizer for lithographic plates.
 Source: slideserve.com
Source: slideserve.com
Values in this range are credible Compliance Guidance. 140067 1007944 140067 1599943 Percent composition by element. The signals of NO mz 30 and NH 3 mz 17 were recorded indicating that NO is one of the intermediates in the NO 3-RR. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Nitrate is a polyatomic ion with the chemical formula NO 3.
 Source: brainly.in
Source: brainly.in
H 1 O 16 N 14 Solution. Used in industries as a viscosity adjuster. Silver nitrate is not expected to burn. What percentage of the mass of ammonium nitrate is nitrogen. The element nitrogen is the active ingredient for agricultural purposes so the mass percentage of nitrogen in the compound is a practical and economic concern for consumers choosing among these fertilizers.

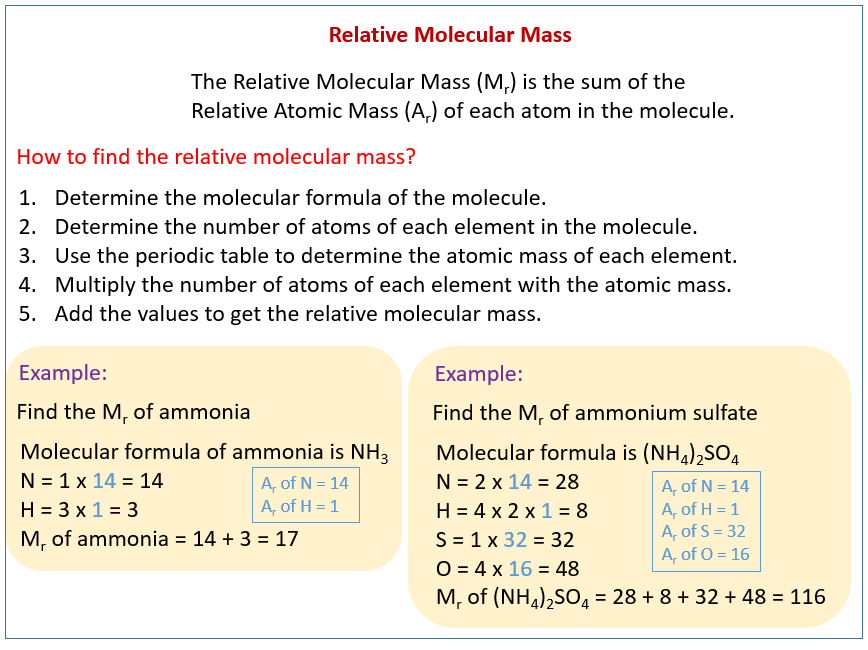
The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 112 of the mass of a neutral carbon-12 12 C isotope atom. Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. However if large quantities are involved in a fire an explosion may result. This compound is also known as Ammonium Nitrate. Mass of nitrogen in the formula 28 Mass of nitrogen as a fraction of the total.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
What percentage of the mass of ammonium nitrate is nitrogen. For network solids the term formula unit is used in stoichiometric calculations. Has been widely used in solid propellants fireworks and flares. Used in the purification of nitric acid. Almost all inorganic nitrates.
 Source: youtube.com
Source: youtube.com
Used to manufacture ammonium nitrate. Atomic Mass of Atoms. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. CSHOs should ask employers how the ammonium nitrate storage area is ventilated in case of a fire. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
 Source: youtube.com
Source: youtube.com
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. MgNO 3 2 Uses Magnesium nitrate Magnesium nitrate is used as a dehydrating agent to prepare concentrated nitric acid. And is a constituent of many munition components. The formula for ammonium nitrate is NH 4 NO 3 Relative atomic masses. Nitrate ions have a molecular mass of 63 and they are comprised of one nitrogen atom linked with three exactly the same oxygen atoms.
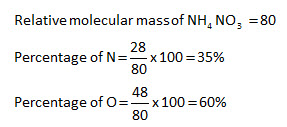 Source: forum.byjus.com
Source: forum.byjus.com
What is relative formula mass and relative molecular mass. As one example consider the common nitrogen-containing fertilizers ammonia NH 3 ammonium nitrate NH 4 NO 3 and urea CH 4 N 2 O. The formula for ammonium nitrate is NH 4 NO 3 Relative atomic masses. Manufacture of ammonium perchlorate began in the 1940s primarily for use by the defense industry and later by the aerospace industry. M contaminant molecular weight lb m lb-mol k mixing coefficient 01 to 10 perfect mixing.
 Source: youtube.com
Source: youtube.com
Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. For molecules with a. The formula for ammonium nitrate is NH 4 NO 3 Relative atomic masses. The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 112 of the mass of a neutral carbon-12 12 C isotope atom. Almost all inorganic nitrates.
 Source: perso.numericable.fr
Source: perso.numericable.fr
Finally differential electrochemical mass spectrometry DEMS was used for in-situ detection of molecular intermediates and products on TiO 2-x Fig. Finally differential electrochemical mass spectrometry DEMS was used for in-situ detection of molecular intermediates and products on TiO 2-x Fig. Mass of nitrogen in the formula 28 Mass of nitrogen as a fraction of the total. The element nitrogen is the active ingredient for agricultural purposes so the mass percentage of nitrogen in the compound is a practical and economic concern for consumers choosing among these fertilizers. Used in the manufacturing of petrochemicals.
 Source: wiredfaculty.com
Source: wiredfaculty.com
As one example consider the common nitrogen-containing fertilizers ammonia NH 3 ammonium nitrate NH 4 NO 3 and urea CH 4 N 2 O. For molecules with a. The signals of NO mz 30 and NH 3 mz 17 were recorded indicating that NO is one of the intermediates in the NO 3-RR. For network solids the term formula unit is used in stoichiometric calculations. Used in the purification of nitric acid.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molecular mass of ammonium nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.