Molecular formula for 2 methylpentane
Home » chemical » Molecular formula for 2 methylpentane >Molecular formula for 2 methylpentane
Molecular Formula For 2 Methylpentane. Which of the following could not be the molecular formula for an alkane molecule. In organic chemistry an alkane or paraffin a historical trivial name that also has other meanings is an acyclic saturated hydrocarbonIn other words an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carboncarbon bonds are single. A carbon atoms C oxygen atoms. The four-carbon alkane is butane with the formula C 4 H 10.
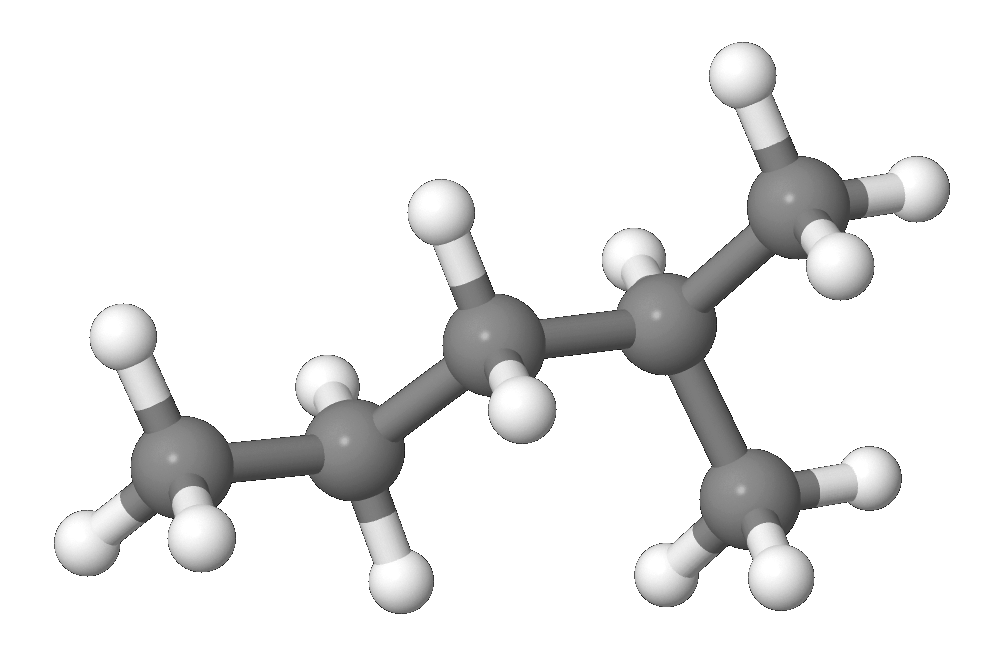 2 Methylpentane Wikipedia From en.wikipedia.org
2 Methylpentane Wikipedia From en.wikipedia.org
Solution i CH3 CH2 CH2 CH2 CH2 CH3 n-Hexane 2-Methylpentane 3-Methylpentane 23-Dimethylbutane 22 - Dimethylbutane Based upon the number of carbon atoms attached to a carbon atom the carbon atom is termed as primary 1 secondary 2. Which of the following hydrocarbons does not have isomers. C have the same molecular formula. Alkanes have the general chemical formula C n H 2n2The alkanes range in complexity from the simplest case of. The calorific value is the total energy released as heat when a substance undergoes complete combustion with oxygen under standard conditionsThe chemical reaction is typically a hydrocarbon or other. A 2-methylpentane b 3.
Anti-Acetyl-CoA Synthetase Antibody clone 6C12.
The formula of the five-carbon alkane pentane is C 5 H 12 so the difference in hydrogen content is 4. 3-Ethyl-2-methylpentane C8H18 CID 11863 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. What is the number of electrons shared in the multiple carbon-carbon bond in one molecule of I -pentyne. The formula of the five-carbon alkane pentane is C 5 H 12 so the difference in hydrogen content is 4. The names formulas and physical properties for a variety of alkanes with the generic formula C n H 2n2 are given in the table below. Solution i CH3 CH2 CH2 CH2 CH2 CH3 n-Hexane 2-Methylpentane 3-Methylpentane 23-Dimethylbutane 22 - Dimethylbutane Based upon the number of carbon atoms attached to a carbon atom the carbon atom is termed as primary 1 secondary 2.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
A C2H2 B C C4H8 D C4H10 30. In this formula N H is the number of hydrogen atoms while N C is the number of carbon atoms in the hydrocarbon molecule. C have the same molecular formula. Empirical Formula Hill Notation. The boiling points of the alkanes gradually increase with the molecular weight of these compounds.
A C6H14 B C6H16 C CH4 D C23H48. Normal alkanes are the chain molecules depicted the first four examples in Table 201Once the number of carbon atoms reaches four butanes different permutations of an alkane molecule can exist that still honor Equation 201 but do not form chain molecules. Alkanes have the general chemical formula C n H 2n2The alkanes range in complexity from the simplest case of. Which of the following could not be the molecular formula for an alkane molecule. Which of the following is a correct alkane structural formula.
 Source: fishersci.se
Source: fishersci.se
The representation CH3-CH2-CH2-CH2-CH3for the alkane pentane is an example of A a molecular formula B a condensed structural formula C an expanded structural formula D a skeletal structural formula. Clonality Application Species Reactivity Citations SDS. A carbon atoms C oxygen atoms. D have a different content of the isotopes of hydrogen. E react vigorously with one another.
 Source: youtube.com
Source: youtube.com
D have a different content of the isotopes of hydrogen. The heating value or energy value or calorific value of a substance usually a fuel or food see food energy is the amount of heat released during the combustion of a specified amount of it. Empirical Formula Hill Notation. A carbon atoms C oxygen atoms. A C6H14 B C6H16 C CH4 D C23H48.
The four-carbon alkane is butane with the formula C 4 H 10. For the preparation of dilute volumetric solutions or for direct use cHCl 5 moll 5 N Combi-Titrisol. Which of the following is a correct alkane structural formula. The calorific value is the total energy released as heat when a substance undergoes complete combustion with oxygen under standard conditionsThe chemical reaction is typically a hydrocarbon or other. The formula of the five-carbon alkane pentane is C 5 H 12 so the difference in hydrogen content is 4.
 Source: youtube.com
Source: youtube.com
Solution i CH3 CH2 CH2 CH2 CH2 CH3 n-Hexane 2-Methylpentane 3-Methylpentane 23-Dimethylbutane 22 - Dimethylbutane Based upon the number of carbon atoms attached to a carbon atom the carbon atom is termed as primary 1 secondary 2. A carbon atoms C oxygen atoms. C 26 H 44 N 7 O 17 P 3 S xLi yH 2 O. Which atoms can bond with each other to fom chains rings 15 H 13 1 c H H B 2-methylpentane D 4-methylpentane or networks. The names formulas and physical properties for a variety of alkanes with the generic formula C n H 2n2 are given in the table below.
 Source: molinstincts.com
Source: molinstincts.com
Alkanes have the general chemical formula C n H 2n2The alkanes range in complexity from the simplest case of. Which of the following could not be the molecular formula for an alkane molecule. Which of the following is a correct alkane structural formula. In this formula N H is the number of hydrogen atoms while N C is the number of carbon atoms in the hydrocarbon molecule. A carbon atoms C oxygen atoms.
 Source: tcichemicals.com
Source: tcichemicals.com
A C6H14 B C6H16 C CH4 D C23H48. For the preparation of dilute volumetric solutions or for direct use cHCl 5 moll 5 N Combi-Titrisol. Clonality Application Species Reactivity Citations SDS. The boiling points of the alkanes gradually increase with the molecular weight of these compounds. Which of the following could not be the molecular formula for an alkane molecule.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Which of the following is a correct alkane structural formula. A 2-methylpentane b 3. As noted earlier in the Analysis of Molecular Formulas section the molecular formula of a hydrocarbon provides information about the possible structural types it may represent. Of alkanes corresponding to the molecular formula C6H14. C have the same molecular formula.
 Source: study.com
Source: study.com
C 26 H 44 N 7 O 17 P 3 S xLi yH 2 O. Write balanced equations for the complete combustion of the following hydrocarbons. The formula shown below. At room temperature the lighter alkanes are gases. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molecular formula for 2 methylpentane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.