Molar mass of copper 2 sulfate pentahydrate
Home » chemical » Molar mass of copper 2 sulfate pentahydrate >Molar mass of copper 2 sulfate pentahydrate
Molar Mass Of Copper 2 Sulfate Pentahydrate. 147g H 2 O x 1 mol H 2 O 1802g mol-1 H 2 O 008158 mol H 2 O - Ratio of Water to Anhydrate. This means that youre going to have to account for the mass of this water of crystallization in. 2 Magnesium sulfate - Determining the Number of Moles. Molar Mass of Frequently Calculated Chemicals.
 Molar Relationships Ppt Download From slideplayer.com
Molar Relationships Ppt Download From slideplayer.com
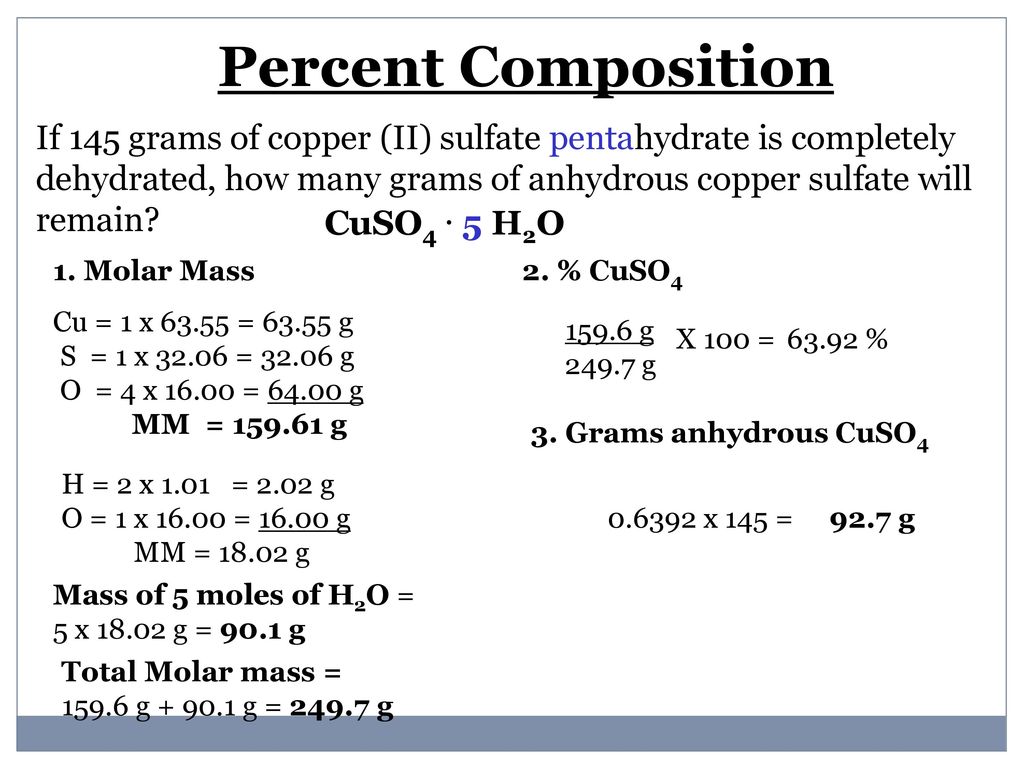
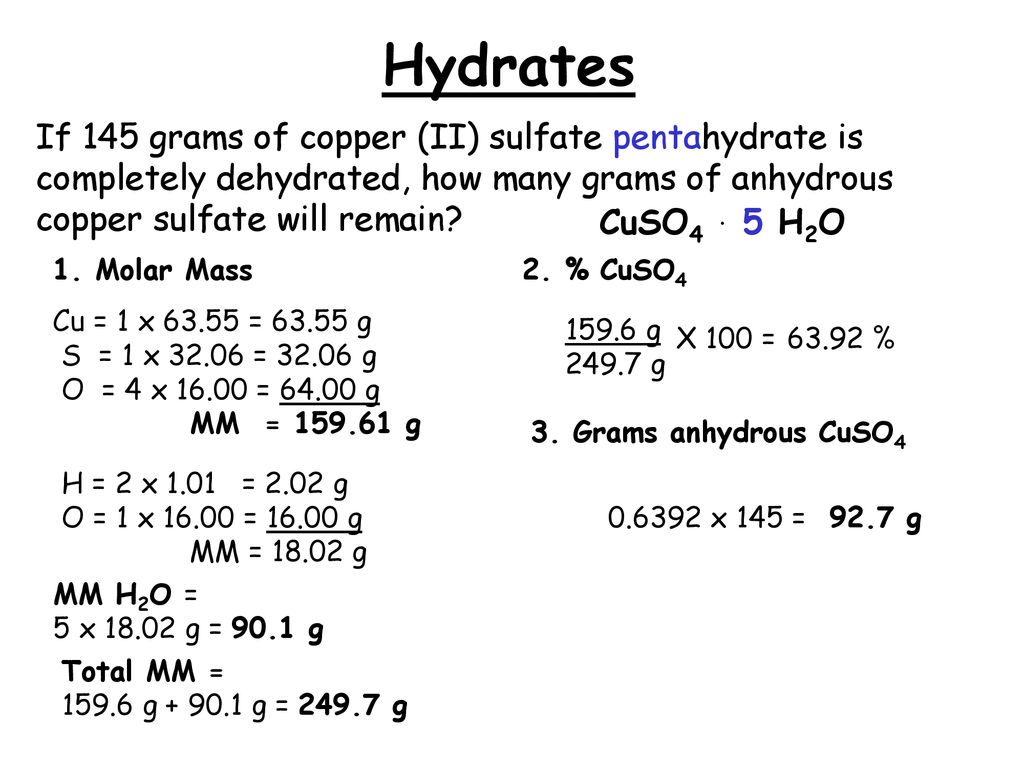
Find the molar mass of copper on the periodic table. And as a food additive with E number of E327Some cheese crystals. 25 g CuSO_4 5H_2O Youre dealing with copperII sulfate pentahydrate CuSO_4 5H_2O an ionic compound that contains water of crystallization in its structure. This means that youre going to have to account for the mass of this water of crystallization in. For best accuracy use the mass measured with the balance during the experiment. A mass in grams of 205 mol chromiumIII sulfate decahydrate 1170 g b mass in grams of 946 10 24 molecules of dichlorine heptaoxide 2870 g c number of moles and formula units in 592 g lithium sulfate moles 0539 mol formula units 324e23 formula units d number of lithium ions sulfate ions S atoms and O atoms in the mass.
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver.
Now the difference between hydrated copperII sulfate and anhydrous copperII sulfate except for the fact that the former contains water of crystallization is the color. The water in these compounds can be removed quantitatively by heating the compound with a bunsen burner. Calcium lactate is a white crystalline salt with formula C 6 H 10 CaO 6 consisting of two lactate anions H 3 C CHOH CO 2 for each calcium cation Ca 2It forms several hydrates the most common being the pentahydrate C 6 H 10 CaO 6 5 H 2 O. 06 gmol Molar mass of SO42- PARTA Mass of unknown metal sulfate hydrate MSH Mass of unknown sample sulfate anhydrous metal sulfate only 10. Is iron sulphide compounds. If chemists want to speak about incredibly small atoms and molecules an amount far greater than a dozen is needed.
 Source: youtube.com
Source: youtube.com
880 to 1. Use a plastic pipette to add a small amount of silver nitrate solution AgNO3 to the copper. Choose the closest answer. The known hydrates and the approximate ranges of temperature and concentration mass percent of NaOH of their saturated water solutions are. Calcium lactate is a white crystalline salt with formula C 6 H 10 CaO 6 consisting of two lactate anions H 3 C CHOH CO 2 for each calcium cation Ca 2It forms several hydrates the most common being the pentahydrate C 6 H 10 CaO 6 5 H 2 O.
 Source: slideplayer.com
Source: slideplayer.com
880 to 1. If chemists want to speak about incredibly small atoms and molecules an amount far greater than a dozen is needed. On the other hand. 2 O which result in a complex solubility diagram that was described in detail by S. Copper II sulfate pentahydrate.
 Source: youtube.com
Source: youtube.com
Calcium lactate is used in medicine mainly to treat calcium deficiencies. The loss in mass of the compound after losing the water can be used to calculate the amount of water originally in the hydrated sample. The known hydrates and the approximate ranges of temperature and concentration mass percent of NaOH of their saturated water solutions are. For best accuracy use the mass measured with the balance during the experiment. Molar mass of.
 Source: youtube.com
Source: youtube.com
Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment. Calcium lactate is a white crystalline salt with formula C 6 H 10 CaO 6 consisting of two lactate anions H 3 C CHOH CO 2 for each calcium cation Ca 2It forms several hydrates the most common being the pentahydrate C 6 H 10 CaO 6 5 H 2 O. Copper II sulfate pentahydrate. And as a food additive with E number of E327Some cheese crystals. Heptahydrate NaOH7 H 2 O.
 Source: slidetodoc.com
Source: slidetodoc.com
MgC 2 O 4 2H 2 O. 25 g CuSO_4 5H_2O Youre dealing with copperII sulfate pentahydrate CuSO_4 5H_2O an ionic compound that contains water of crystallization in its structure. A mass in grams of 205 mol chromiumIII sulfate decahydrate 1170 g b mass in grams of 946 10 24 molecules of dichlorine heptaoxide 2870 g c number of moles and formula units in 592 g lithium sulfate moles 0539 mol formula units 324e23 formula units d number of lithium ions sulfate ions S atoms and O atoms in the mass. MgCO 3 3H 2 O. The water in these compounds can be removed quantitatively by heating the compound with a bunsen burner.
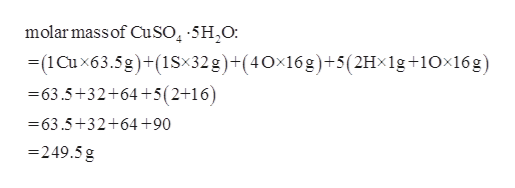 Source: bartleby.com
Source: bartleby.com
Given that argon is relatively inert which of. MgCO 3 5H 2 O. Heptahydrate NaOH7 H 2 O. A mass in grams of 205 mol chromiumIII sulfate decahydrate 1170 g b mass in grams of 946 10 24 molecules of dichlorine heptaoxide 2870 g c number of moles and formula units in 592 g lithium sulfate moles 0539 mol formula units 324e23 formula units d number of lithium ions sulfate ions S atoms and O atoms in the mass. On the other hand.
 Source: youtube.com
Source: youtube.com
Mg 3 PO 4 2. From 28 C 188 to 24 C 222. Repeat the above procedure with copperII sulfate pentahydrate and again with potassium aluminum sulfate. Washing soda is a hydrated compound whose formula can be written Na 2 CO 3 xH2O. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver.
 Source: youtube.com
Source: youtube.com
The loss in mass of the compound after losing the water can be used to calculate the amount of water originally in the hydrated sample. Use a plastic pipette to add a small amount of silver nitrate solution AgNO3 to the copper. For example copperII pentahydrate CuSO_4 5H_2O is blue. Copper sulfate pentahydrate CuSO45H2O or CuH10O9S CID 24463 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. 06 gmol Molar mass of SO42- PARTA Mass of unknown metal sulfate hydrate MSH Mass of unknown sample sulfate anhydrous metal sulfate only 10.
 Source: slideplayer.com
Source: slideplayer.com
Is iron sulphide compounds. MgC 2 O 4 2H 2 O. Divide the mass of water in one mole. From 28 C 188 to 24 C 222. The loss in mass of the compound after losing the water can be used to calculate the amount of water originally in the hydrated sample.
 Source: youtube.com
Source: youtube.com
25 g CuSO_4 5H_2O Youre dealing with copperII sulfate pentahydrate CuSO_4 5H_2O an ionic compound that contains water of crystallization in its structure. A mass in grams of 205 mol chromiumIII sulfate decahydrate 1170 g b mass in grams of 946 10 24 molecules of dichlorine heptaoxide 2870 g c number of moles and formula units in 592 g lithium sulfate moles 0539 mol formula units 324e23 formula units d number of lithium ions sulfate ions S atoms and O atoms in the mass. In the case of copperII sulfate hydrates you only get x as a whole number. 2 O which result in a complex solubility diagram that was described in detail by S. Mg 3 PO 4 2.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molar mass of copper 2 sulfate pentahydrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.