Molar mass lithium hydroxide
Home » chemical » Molar mass lithium hydroxide >Molar mass lithium hydroxide
Molar Mass Lithium Hydroxide. Calculate the volume of 100 grams of Hydrochloric acid. A patient takes two teaspoons 5 mL each of syrup. The concentration of aluminium hydroxide in the syrup is 50 mg mL 1. A solution with a volume of 025 liters contains 20 grams of hydrogen fluoride HF.
 Lithium Hydroxide Lioh Physical And Chemical Properties Uses From byjus.com
Lithium Hydroxide Lioh Physical And Chemical Properties Uses From byjus.com
In the decomposition of potassium chlorate how many moles of potassium chlorate are needed to produce 50 moles of oxygen gas. 3 If I add the two reactions I obtain this. Lithium aluminium hydride LiAlH4 or AlH4Li CID 21226445 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Concentration of Hydrochloric acid. 355 grams b I actually produced 6 grams of lithium chloride. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS.
The ground state electronic configuration of.
37 by mass ww Density of 37 Hydrochloric acid. A histological examination of. Lithium aluminium hydride LiAlH4 or AlH4Li CID 21226445 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. The ground state electronic configuration of. Concentration of Hydrochloric acid. However the solubility of calcium hydroxide is very low.
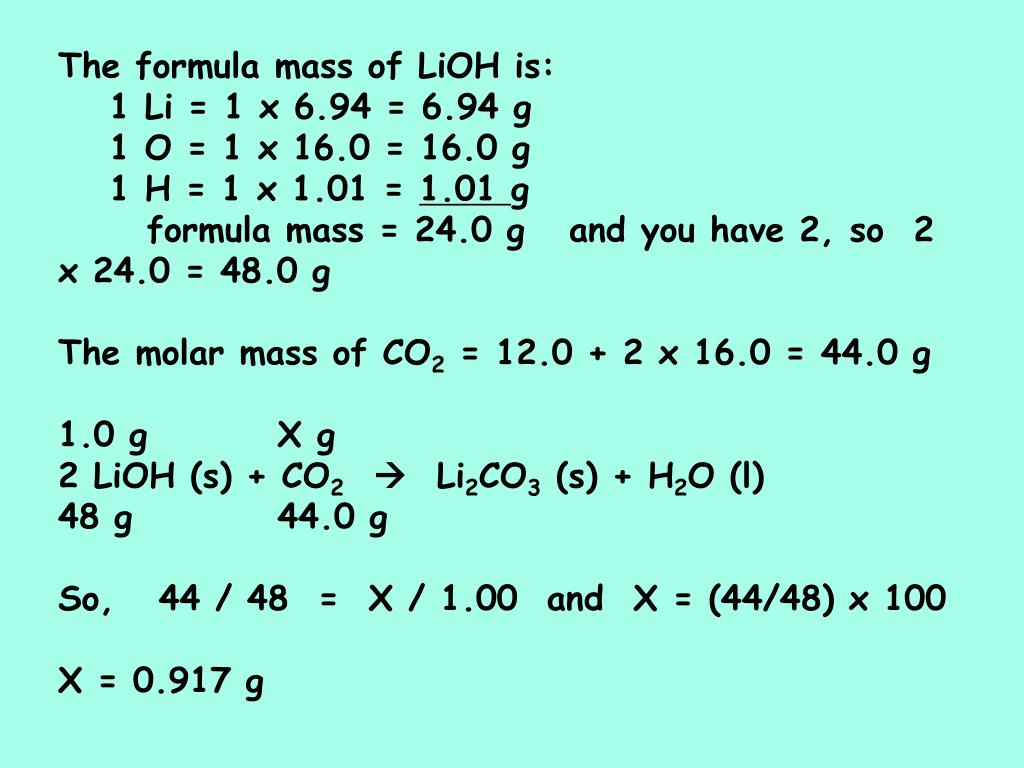 Source: slideserve.com
Source: slideserve.com
The ground state electronic configuration of. 37 by mass ww Density of 37 Hydrochloric acid. Wishing for a unique insight into a subject matter for your subsequent individual research. A solution with a volume of 025 liters contains 20 grams of hydrogen fluoride HF. 8 1700971 2018.
 Source: msrblog.com
Source: msrblog.com
A solution with a volume of 025 liters contains 20 grams of hydrogen fluoride HF. Molar mass is used to describe the mass of one mole of a chemical compound. How many moles are in 983 grams of aluminum hydroxide AlOH 3. Aluminium atoms have 13 electrons and the shell structure is 283. 12601 moles 126 moles 11 How many grams are in 002 moles of beryllium iodide BeI 2.
 Source: slideplayer.com
Source: slideplayer.com
8 1700971 2018. In the decomposition of potassium chlorate how many moles of potassium chlorate are needed to produce 50 moles of oxygen gas. A solution with a volume of 025 liters contains 20 grams of hydrogen fluoride HF. The molar mass of H 2 SO 4 and SO 4 is 981 g mol 1 and 961 g mol 1. Aluminium hydroxide AlOH 3 is used as an antacida chemical used to treat acidity.
 Source: chemistrysaaangus.weebly.com
Source: chemistrysaaangus.weebly.com
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. The octahydrate which is simple to prepare is white in contrast. Wishing for a unique insight into a subject matter for your subsequent individual research. This metal peroxide exists in several hydrates and peroxyhydrates including Na 2 O 2 2H 2 O 2 4H 2 O Na 2 O 2 2H 2 O Na 2 O 2 2H 2 O 2 and Na 2 O 2 8H 2 O.
 Source: youtube.com
Source: youtube.com
How many moles are in 983 grams of aluminum hydroxide AlOH 3. What is my theoretical yield of lithium chloride. Group in periodic table. Aluminium hydroxide AlOH 3 is used as an antacida chemical used to treat acidity. A solution with a volume of 025 liters contains 20 grams of hydrogen fluoride HF.
 Source: chegg.com
Source: chegg.com
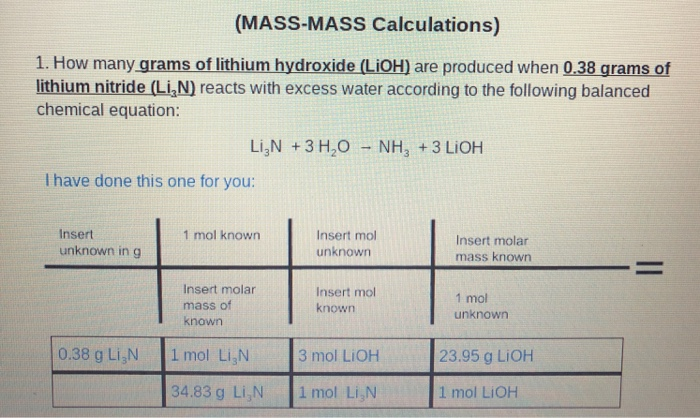
How many moles are in 983 grams of aluminum hydroxide AlOH 3. M r of LiOH 24 molar volume 24 dm 3 Step 1 - Calculate the amount of carbon dioxide. What is my percent yield. When ceCaOH2 dissolves in water the ionization reaction is as follows. Both are available commercially.

Molar mass is used to describe the mass of one mole of a chemical compound. Volume Weight Density. 37 by mass ww Density of 37 Hydrochloric acid. While classified as a strong base lithium hydroxide is the weakest known alkali metal hydroxide. Group in periodic table.
 Source: en.wikipedia.org
Source: en.wikipedia.org
However the solubility of calcium hydroxide is very low. We provide solutions to students. Converting Percentage by Mass to Molarity. Relative to the number of strong acids there are fewer number of strong bases and most are alkali hydroxides. Molar mass of lithium is 694 gmole 15 grams x 1 mole 21614 moles lithium 694grams 2 How.
 Source: fishersci.co.uk
Source: fishersci.co.uk
We need to know the molar relationship between Li and CO 2. None Period in periodic table. Note that two LiOH and one H 2 O. Converting Percentage by Mass to Molarity. Aluminium atoms have 13 electrons and the shell structure is 283.
 Source: byjus.com
Source: byjus.com
Calculate the volume of 100 grams of Hydrochloric acid. 169 2 C 3 H 8 5 O 2 3 CO 2 4 H 2 O a If I start with 5 grams of C 3 H 8 what is my theoretical yield. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS. The levels in the lithium 1 mEqkg and 2 mEqkg groups were almost the same and were higher than the control group. What is my theoretical yield of lithium chloride.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar mass lithium hydroxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.