Methyl propyl and water react
Home » chemical » Methyl propyl and water react >Methyl propyl and water react
Methyl Propyl And Water React. The hydrogens bonded to the aromatic ring referred to as phenyl hydrogens above have relatively high bond dissociation energies and are not substituted. Since the alcoholic carbon is connected to three other carbons it is tertiary hence the prefix tert It is used as a solvent a denaturant for ethanol as an octane booster in gasoline and in some pain thinners. Compounds RNH 2 are called primary amines R 2 NH secondary amines and R 3 N are tertiary amines. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español.
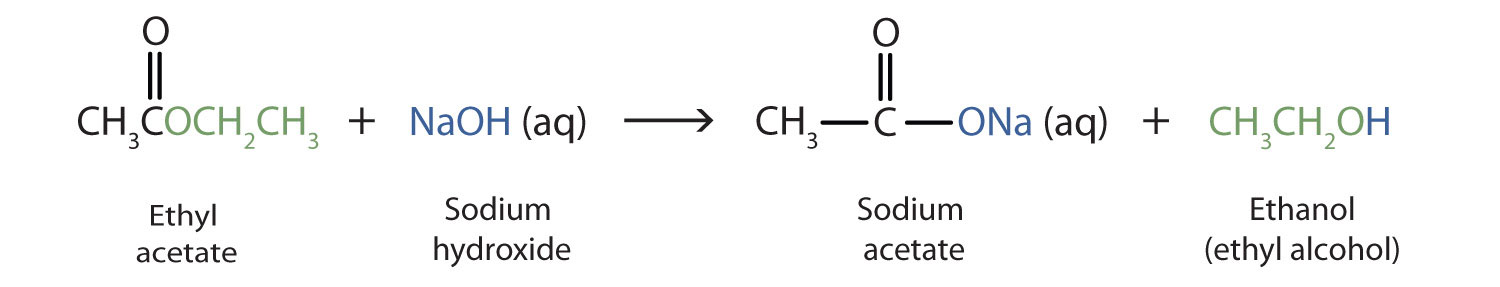 15 9 Hydrolysis Of Esters Chemistry Libretexts From chem.libretexts.org
15 9 Hydrolysis Of Esters Chemistry Libretexts From chem.libretexts.org
Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Compounds RNH 2 are called primary amines R 2 NH secondary amines and R 3 N are tertiary amines. Benzoates are most effective when undissociated. It is important to keep in mind that alcohols can react with isocyanates and thus can interfere with the drying process of such coatings. Solution The longest carbon chain runs horizontally across the page and contains six carbon atoms this makes the base of the name hexane but we will also need to incorporate the name of the branch. Concentrated sulfuric acid is a catalyst for this reaction.
This effect can be reduced by using secondary or tertiary alcohols.
Methyl isobutyl Ketone a. The compound forms colloids when dissolved in water. These include lactic acid and lactates propionic acid and propionates citric acid acetic acid sorbic acid and sorbates benzoic acid and benzoates and methyl and propyl parabens benzoic acid derivatives. Add 2 mL of 5 NaOH solution and then introduce the potassium iodide - iodine reagent dropwise with shaking until a definite dark colour of iodine persists. 3-Methyl-2-butanone C5H10O CID 11251 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Allow to stand for 2 - 3 minutes.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is important to keep in mind that alcohols can react with isocyanates and thus can interfere with the drying process of such coatings. This effect can be reduced by using secondary or tertiary alcohols. Therefore they require low pH values for activity 2540. Since the alcoholic carbon is connected to three other carbons it is tertiary hence the prefix tert It is used as a solvent a denaturant for ethanol as an octane booster in gasoline and in some pain thinners. Allow to stand for 2 - 3 minutes.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Ethanol a high evaporation solvent able to. This effect can be reduced by using secondary or tertiary alcohols. The compound forms colloids when dissolved in water. The sodium salt of benzoate is used to improve solubility in foods. CH 3 OH aq C 3 H 7 CO 2 H aq C 3 H 7 CO 2 CH 3.
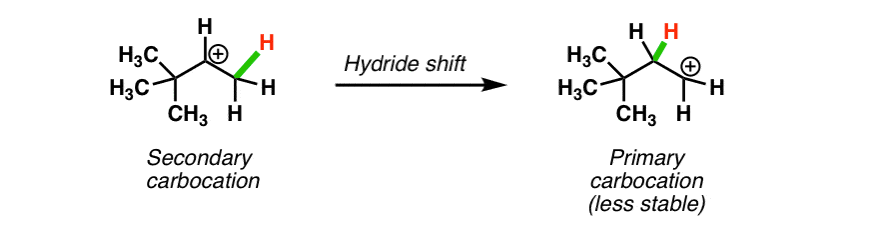 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
These include lactic acid and lactates propionic acid and propionates citric acid acetic acid sorbic acid and sorbates benzoic acid and benzoates and methyl and propyl parabens benzoic acid derivatives. In another study tap water at 20 C 68 F containing 50200 μM free chlorine was spiked with 05 μM propylparaben and the composition of the mixture was monitored over 40. This effect can be reduced by using secondary or tertiary alcohols. The designation of amines as primary secondary and tertiary is different from the usage of these terms in. 3-Methyl-2-butanone C5H10O CID 11251 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
 Source: chem.libretexts.org
Source: chem.libretexts.org
3-Methyl-2-butanone C5H10O CID 11251 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Ethers such as METHYL PROPYL ETHER can act as bases. The compound forms colloids when dissolved in water. Naming Substituted Alkanes Name the molecule whose structure is shown here. Butanoic acid will react with alcohols in the presence of concentrated sulfuric acid to form esters.
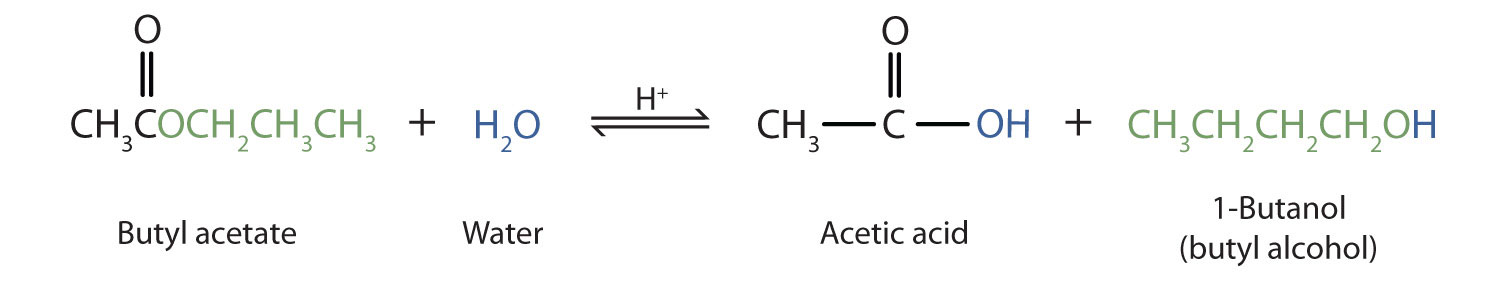 Source: chem.libretexts.org
Source: chem.libretexts.org
If no iodoform separates at room temperature warm the. Since the alcoholic carbon is connected to three other carbons it is tertiary hence the prefix tert It is used as a solvent a denaturant for ethanol as an octane booster in gasoline and in some pain thinners. Benzoates are most effective when undissociated. Compounds RNH 2 are called primary amines R 2 NH secondary amines and R 3 N are tertiary amines. Ethers may react violently with strong oxidizing agents.
 Source: chegg.com
Source: chegg.com
The Arrhenius equation was used in a study to calculate activation energies for the chlorination of four parent parabens methyl- ethyl- propyl- and butylparaben and was found to range from 3647 kJmol. Therefore they require low pH values for activity 2540. The hydrogens bonded to the aromatic ring referred to as phenyl hydrogens above have relatively high bond dissociation energies and are not substituted. Dissolve 01 g or 5 drops of the compound in 2 mL of water. 2-Methyl-2-propanol or tert-butanol or tert-butyl alcohol or t-butyl alcohol is a three-carbon chain with the OH group and a methyl group on the middle carbon.
 Source: nagwa.com
Source: nagwa.com
Therefore they require low pH values for activity 2540. CH 3 OH aq C 3 H 7 CO 2 H aq C 3 H 7 CO 2 CH 3. Ethers may react violently with strong oxidizing agents. Dissolve 01 g or 5 drops of the compound in 2 mL of water. Small alcohols are soluble in water but miscibility falls off as the hydrocarbon chain length becomes longer.
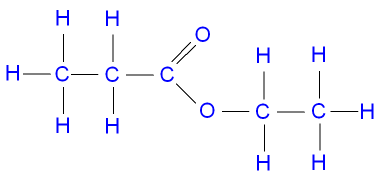 Source: gcsescience.com
Source: gcsescience.com
3-Methyl-2-butanone C5H10O CID 11251 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. They form salts with strong acids and addition complexes with Lewis acids. The designation of amines as primary secondary and tertiary is different from the usage of these terms in. Methanol butanoic acid methyl butanoate water. Therefore they require low pH values for activity 2540.
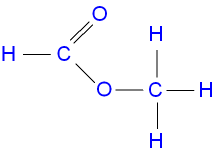 Source: gcsescience.com
Source: gcsescience.com
Therefore they require low pH values for activity 2540. Ethers such as METHYL PROPYL ETHER can act as bases. It is important to keep in mind that alcohols can react with isocyanates and thus can interfere with the drying process of such coatings. 2-Methyl-2-propanol or tert-butanol or tert-butyl alcohol or t-butyl alcohol is a three-carbon chain with the OH group and a methyl group on the middle carbon. CH 3 OH aq C 3 H 7 CO 2 H aq C 3 H 7 CO 2 CH 3.
Concentrated sulfuric acid is a catalyst for this reaction. This effect can be reduced by using secondary or tertiary alcohols. The designation of amines as primary secondary and tertiary is different from the usage of these terms in. The compound forms colloids when dissolved in water. Dissolve 01 g or 5 drops of the compound in 2 mL of water.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title methyl propyl and water react by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.