Mercury i chloride
Home » chemical » Mercury i chloride >Mercury i chloride
Mercury I Chloride. The red form has water in it. Acute exposure guideline levels AEGLs describe the human health effects from once-in-a-lifetime or rare exposure to airborne chemicals. It has a reputation of being very stable and robust. Mercuric chloride was used to disinfect wounds by Arab physicians in the Middle Ages but modern medicine has since deemed it unsafe for use.
 Mercury I Chloride Wikiwand From wikiwand.com
Mercury I Chloride Wikiwand From wikiwand.com
Limits of detection range from 50 glitre for colorimetry to 5 mglitre for titration 6. SilverSilver Chloride AgAgCl - The AgAgCl reference electrode is simpler in construction than the SCE and it contains no mercury. USCG 1999 CAMEO Chemicals. Once in the bays sediments the mercury was readily absorbed by marine species contaminating the entire ecosystem. It contains cobalt and chloride ions. Plants contain various amount of chlorine.
It can be heated to turn it into the blue form without water.
Mercury used in the Caster-Kellner process contaminates the products and is an environmental hazard due to sublimation. USCG 1999 CAMEO Chemicals. People who eat these fish may be exposed to this form of mercury. Lead II chloride PbCl2 47. SilverSilver Chloride AgAgCl - The AgAgCl reference electrode is simpler in construction than the SCE and it contains no mercury. It can be heated to turn it into the blue form without water.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
CobaltII chloride is normally found in the red or pink form. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. Lead II chloride PbCl2 47. Lithium phosphide Li3P 41. Methyl mercury and other organic mercury compounds are products of reactions between mercury and carbon-based organic compounds.
 Source: en.wikipedia.org
Source: en.wikipedia.org
To ensure that there is no longer Pb 2 is useful an indirect assay. Lead II chloride PbCl2 47. Methyl mercury and other organic mercury compounds are products of reactions between mercury and carbon-based organic compounds. EPA sets legal limits on over 90 contaminants in drinking water. It is a very stable reference electrode.

Limits of detection range from 50 glitre for colorimetry to 5 mglitre for titration 6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE Air Exposure to chloride in air has been reported to be negligible 4. Often brilliantly colored these products soon found widespread. It contains cobalt and chloride ions. Mercury I chloride Hg2Cl2 33.

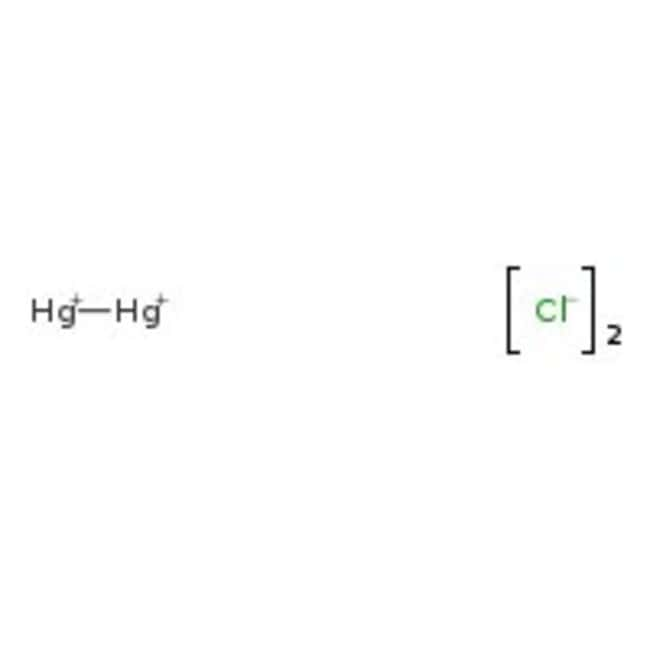
For example in the 1950s the Chisso Corporation in Minamata Japan released untreated effluent containing methyl mercury chloride into Minamata Bay. Other common mercury compounds include mercuric chloride HgCl 2 a highly poisonous salt and that was once used as a wound disinfectant. Blood is primarily used to identify a high level of methyl mercury. MercuryI chloride is the chemical compound with the formula Hg 2 Cl 2. Iron II fluoride FeF2 37.
 Source: wikiwand.com
Source: wikiwand.com
Mercury chloride HgCl2 is a highly toxic compound that volatizes slightly at ordinary temperature and appreciably at 100 degrees C. This suggestion has not been followed but the Stock system remains in use world-wide. EPA sets legal limits on over 90 contaminants in drinking water. It is a very stable reference electrode. People who eat these fish may be exposed to this form of mercury.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Mercury chloride Hg2Cl2 More. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE Air Exposure to chloride in air has been reported to be negligible 4. The upper limit of tolerance varies according. Mercurous chloride is an odorless white solid.
 Source: britannica.com
Source: britannica.com
The red form has water in it. Its chemical formula is CoCl 2. The average in top soils is about 10 ppm. MercuryI chloride is the chemical compound with the formula Hg 2 Cl 2. MercuryII chloride or mercuric chloride historically also known as corrosive sublimate is the chemical compound of mercury and chlorine with the formula HgCl 2.
 Source: fishersci.se
Source: fishersci.se
Limits of detection range from 50 glitre for colorimetry to 5 mglitre for titration 6. It is corrosive to mucous membranes and used as a topical antiseptic and disinfectant. The upper limit of tolerance varies according. No less an authority than Robert Koch championed the use of mercury chloride as an antiseptic although the products propensity to cause tissue irritation limited its use. The red form has water in it.
 Source: youtube.com
Source: youtube.com
It is a component of reference electrodes in electrochemistry. For example FeCl 2which would have been named iron2-chloride according to Stocks original idea became ironII chloride in the revised proposal. The name calomel is thought to come from the Greek καλός beautiful. Growth suffers if the amount of chloride in the soil fall below 2 ppm but it rarely happens. The upper limit of tolerance varies according.
 Source: excichem.com
Source: excichem.com
In 1934 Stock approved of the Roman numerals but felt it better to keep the hyphen and drop the parenthesis. Dimercury dichloride is a mercury coordination entity. So if there is Pb 2 and then chloride Cl-ions in significant. It is corrosive to mucous membranes and used as a topical antiseptic and disinfectant. It has a reputation of being very stable and robust.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title mercury i chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.