Mass of ch2cl2
Home » chemical » Mass of ch2cl2 >Mass of ch2cl2
Mass Of Ch2cl2. - The mass number is given by the sum of the protons and neutrons in the nucleus. It will largely depend on the type of chemical. Dichloromethane is a member of the class of chloromethanes that is methane in which two of the hydrogens have been replaced by chlorineA dense non-flammible colourless liquid at room temperature bp. Protein Mass Spectrometry and HPLC.
Natural sources of dichloromethane include oceanic sources macroalgae wetlands and volcanoes. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. It will largely depend on the type of chemical. Protein Mass Spectrometry and HPLC. What types of gloves protect your hands from hazardous chemicals. Protein Gel Stains PAGE Dye-Based Protein Gel Stains.
Mass the Styrofoam cup create a data table and record the measurement in your table.
Mass of one atom of an element relative to one twelfth of the mass of one atom of Carbon-12 Relative molecular mass Mr. Corrosiveness is a primary consideration. According to the VSEPR theory the CHCl3 molecule possesses tetrahedral molecular geometry. - The mass number is given by the sum of the protons and neutrons in the nucleus. When working out the vv of a solution the same method is used except it is the volume of the solute ml that is. Molecular mass and formula mass Molecular mass is the sum of atomic masses of the elements present in a molecule.
 Source: researchgate.net
Source: researchgate.net
Rank the following elements. However the majority of dichloromethane in the environment is. Corrosiveness is a primary consideration. Sum of relative atomic masses of all the atoms in one molecule of the compound 45 The Mole Concept A mole of a substance is the amount that contains the same number of units as the number of carbon atoms in 12 grams of carbon-12 A mole. Concentration can be expressed in two ways.
Source: webbook.nist.gov
The type of gloves that you should use should be suitable to the chemicals you are handling. Start with the first step at the top of the list. Electrophoresis Sample Preparation Reagents and Kits. Electrophoresis Western Blotting and ELISA. If you place CH2Cl2 and CHCl3 on a cartesian diagram so that the overall dipole would point to a value of -Y straight down traditionally then the C-Cl bonds have larger -Y values for CH2Cl2 than for CHCl3.
 Source: softschools.com
Source: softschools.com
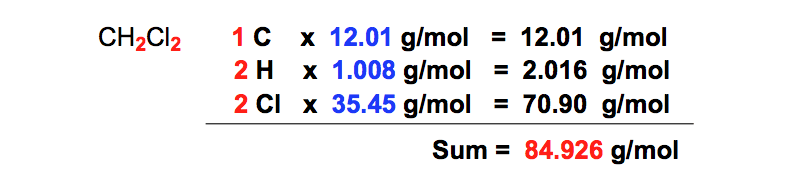
The net dipole moment of CCl4 is zero because four CCl bond dipoles cancel each other. Which of the following reflects the correct procedure to calculate the molar mass of CH2Cl2. Reactions of Alcohols OH PCC CH2Cl2 Na2Cr207 H2SO4 H2O Cr03 H2SO4 H2O PCC CH2Cl2 OH Cr03 H2SO4 H2O 0 Na2Cr2O7 H2SO4 H2O PCC CH2Cl2 16. 120 2102355850 gmol. Theoretical Yield of Caffeine.

Dichloromethane is a member of the class of chloromethanes that is methane in which two of the hydrogens have been replaced by chlorineA dense non-flammible colourless liquid at room temperature bp. Start with the first step at the top of the list. The type of gloves that you should use should be suitable to the chemicals you are handling. 120 2102355850 gmol. According to the VSEPR theory the CHCl3 molecule possesses tetrahedral molecular geometry.
 Source: molinstincts.com
Source: molinstincts.com
Mass of carbon in 1 mol Al2CO33 3 12 g 36 g percentage of carbon in Al2CO33 234 36 100 1538. Which of the following options correctly describe the mass number of an element. Mole concept One mole is the amount of a substance that contains as many particles or entities as. Protein Mass Spectrometry and HPLC. Amino Acid Analysis Reagents and Accessories.
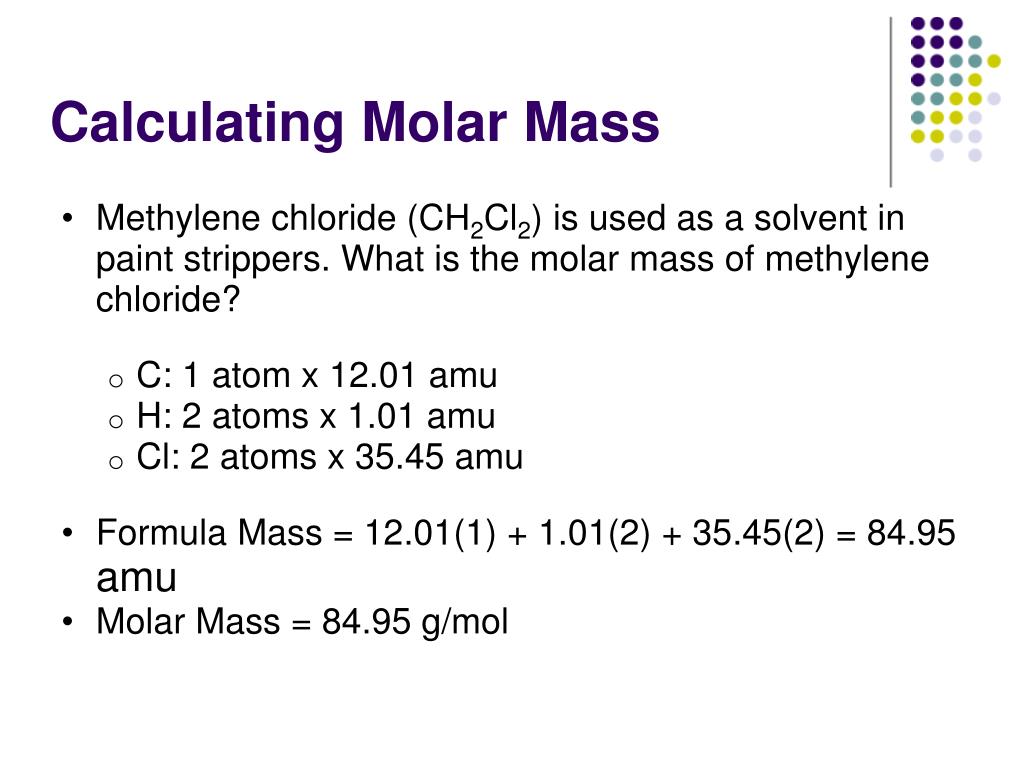 Source: slideserve.com
Source: slideserve.com
Actual Yield of Caffeine. According to the VSEPR theory the CHCl3 molecule possesses tetrahedral molecular geometry. Density of CH2Cl2. This tells us that there is a nitric acid solution of 65 wv. The most commonly used recrystallization solvents are presented in the following table.
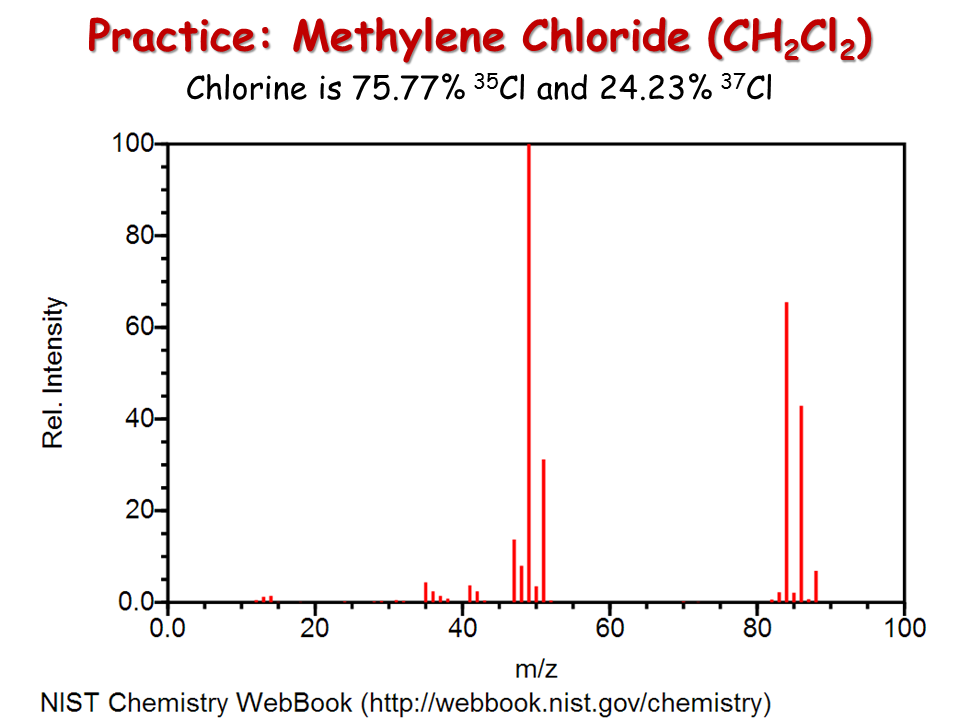
It will largely depend on the type of chemical. 1 mole of this substance contains 1 mol of carbon and 4 moles of chlorine. Weight of 50 mL beaker and boiling stones. Select all that apply. If you place CH2Cl2 and CHCl3 on a cartesian diagram so that the overall dipole would point to a value of -Y straight down traditionally then the C-Cl bonds have larger -Y values for CH2Cl2 than for CHCl3.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
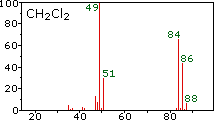
Protein Molecular Weight Markers. The alcohol and chromic acid form a chromate ester that either reacts intramolecularly or intermolecularly in the presence of a base water to yield the corresponding carbonyl compound. Density of CH2Cl2. 1818 Figure 3. How many moles of CH2Cl2 contain 7224 moles of chlorine.
 Source: clutchprep.com
Source: clutchprep.com
Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. The type of gloves that you should use should be suitable to the chemicals you are handling. Rank the following elements. Protein Mass Spectrometry and HPLC. The alcohol and chromic acid form a chromate ester that either reacts intramolecularly or intermolecularly in the presence of a base water to yield the corresponding carbonyl compound.
 Source: slideplayer.com
Source: slideplayer.com
Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. What types of gloves protect your hands from hazardous chemicals. 1 mole of this substance contains 1 mol of carbon and 4 moles of chlorine. Mass of one atom of an element relative to one twelfth of the mass of one atom of Carbon-12 Relative molecular mass Mr. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title mass of ch2cl2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.