Magnesium nitrate solubility
Home » chemical » Magnesium nitrate solubility >Magnesium nitrate solubility
Magnesium Nitrate Solubility. What is the maximum concentration of chromate ion that is allowed before silver chromate will precipitate if the silver ion concentration is 78 x 10-4 M. The nitrate compounds can form a flammable mixture when combined with hydrocarbons. This is our newest publication and has been created to support the school technician profession in Scotland. Sulphate of potash magnesium 3.
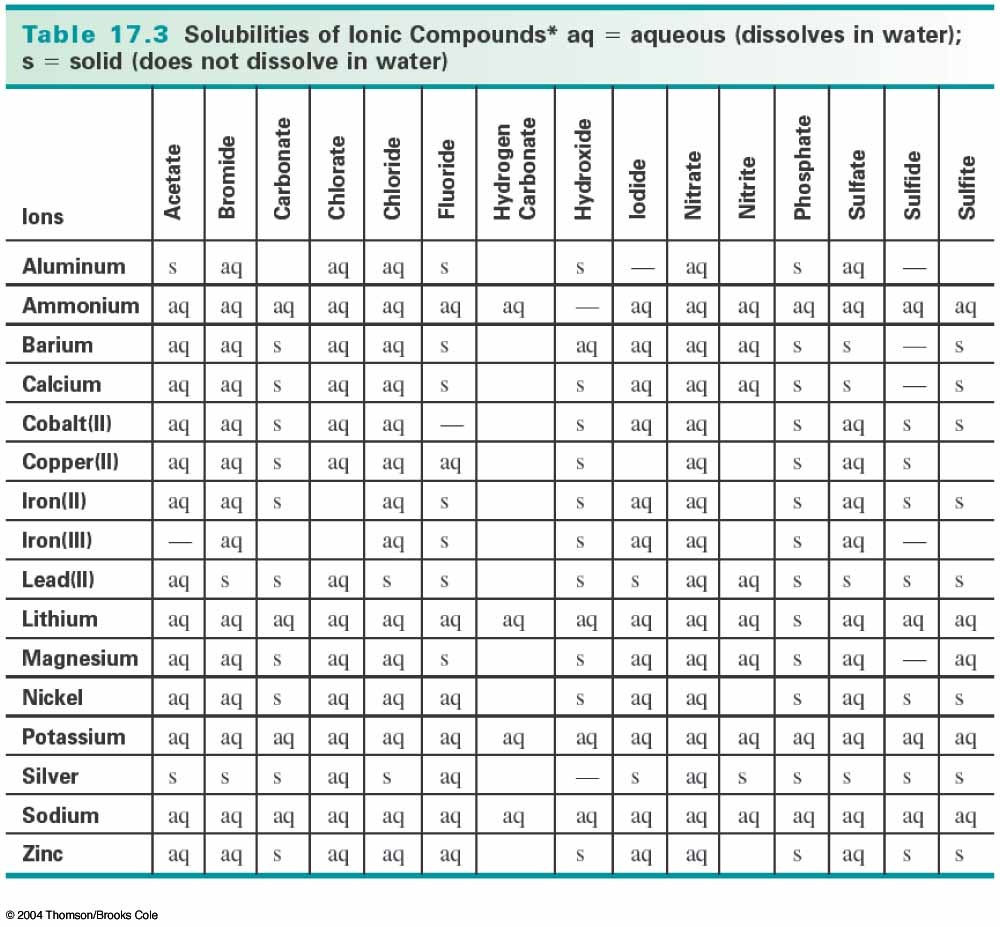 Precipitate Reactions Pms Techno Review From pmstechnoreview.weebly.com
Precipitate Reactions Pms Techno Review From pmstechnoreview.weebly.com
The Manufacturing Process Processing rock salt 1 Underground salt deposits are usually discovered by prospectors searching for water or oil. Magnesium nitrate is a crystalline source with high water solubility for use consistent with nitrates and lower acidic pH. It may be determined by direct measure-ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states. Solubility of nitrogen and nitrogen compounds. The molar solubility of a substance is the number of moles that dissolve per liter of solution. When a salt such as sodium chloride NaCl dissolves in water the ions that make up the salt disperse in the solution but only up to a certain limit called the solubility.
NH 4 Cls 39.
Sulphate of potash magnesium 3. The crystal structure of AgNO 3 is orthorhombic. Nitrogen I oxide solubility is 12 gL and nitriloacetate salt solubility is 640 gL whereas nitrogen chloride is water insoluble. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. C 6 H 12 O 6 l 45. Magnesium nitrate reacts with alkali metal hydroxide to form the corresponding nitrate.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Cd 3 AsO 4 2. CuSO 4 s 14. PbNO 3 2 s 60. The nitrate compounds can form a flammable mixture when combined with hydrocarbons. Magnesium acetate is commonly used as a source of magnesium or for the acetate ion in chemistry experiments.
 Source: youtube.com
Source: youtube.com
In the absence of magnesium the amount of iron required to achieve 50 saturation of the binding sites was 000016 M whereas when magnesium was added only about one third as much iron 54 uM was. Nitrogen N 2 solubility at 20 o C and pressure 1 bar is approximately 20 mgL. Taste thresholds for sodium chloride and calcium chloride in water are in the range 200300 mglitre 2. Organic Ions and Compounds. The reaction between silver nitrate and.
 Source: smart-fertilizer.com
Source: smart-fertilizer.com
AgNO 3 s 245. Melting point - the temperature at which a solid turns into a liquid. Cd 3 AsO 4 2. A mixture consisting of barium dioxide barium nitrate magnesium and zinc exploded from an unknown cause demolishing a small plant. Even the nitrate products are oxidizing agents.
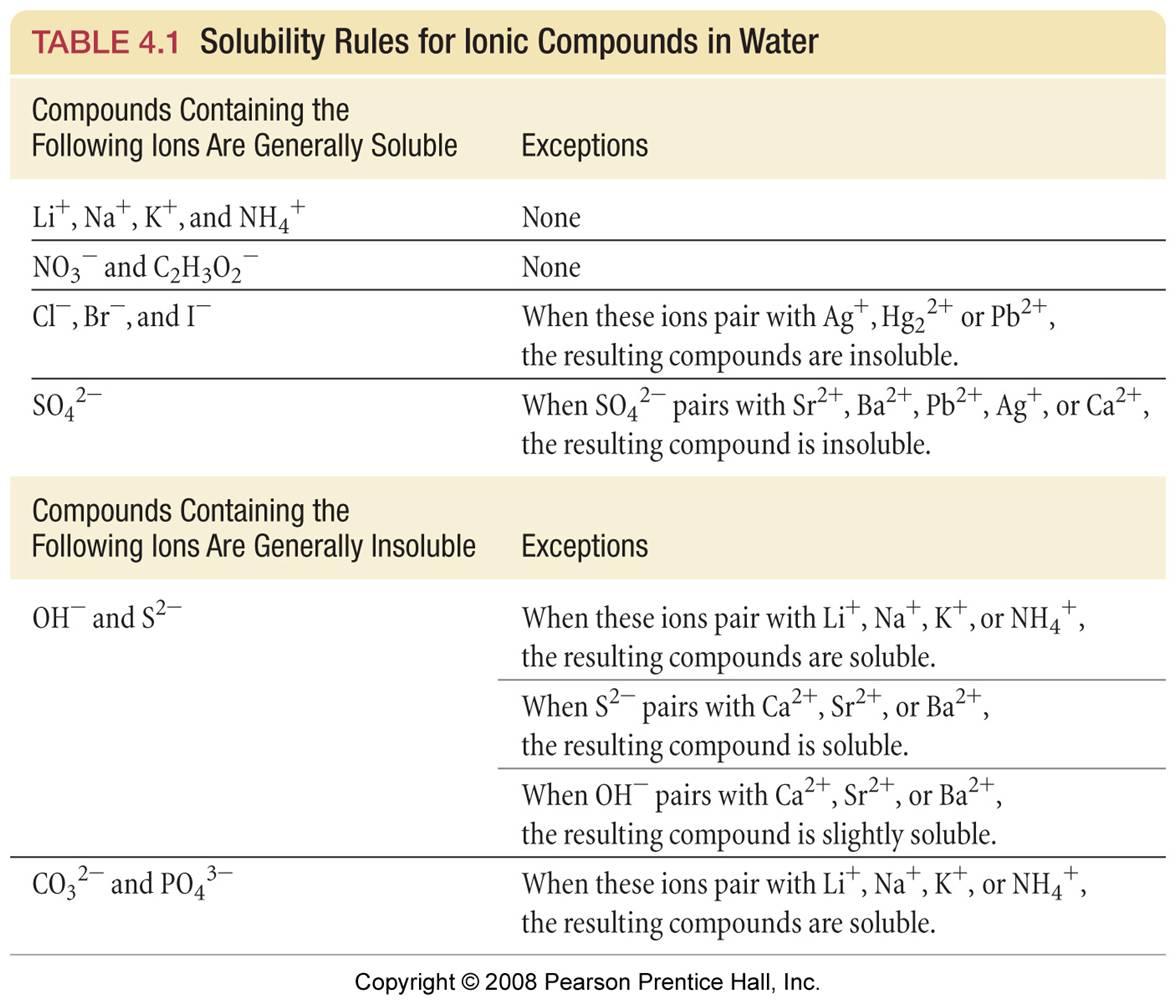 Source: socratic.org
Source: socratic.org
What is the maximum concentration of chromate ion that is allowed before silver chromate will precipitate if the silver ion concentration is 78 x 10-4 M. Sulphate of potash magnesium 3. One example of this is when magnesium acetate and magnesium nitrate were both used to perform molecular dynamics simulations and surface tension measurements. MgSO 4 s 18. Will a precipitate form if 34 mL of 88 x.
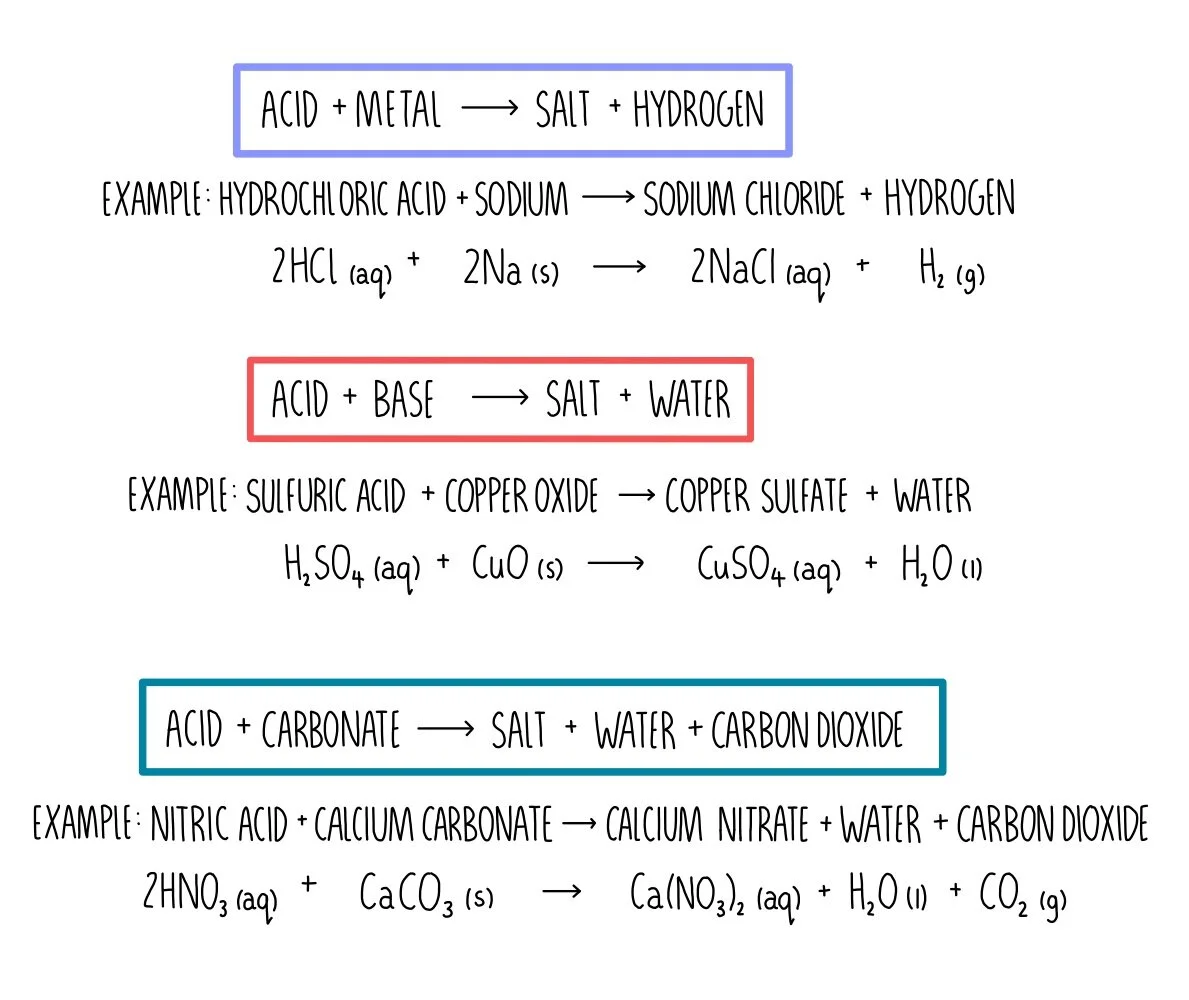 Source: thesciencehive.co.uk
Source: thesciencehive.co.uk
Are the products soluble in water. The balanced reaction would be. If we try to produce a solution where that limit is exceeded the ions will combine to form the solid salt. MgSO 4 s 18. MgNO 3 2 2 NaOH MgOH 2 2 NaNO 3.
 Source: greenhousegrower.com
Source: greenhousegrower.com
One example of this is when magnesium acetate and magnesium nitrate were both used to perform molecular dynamics simulations and surface tension measurements. Ksp solubility product constants of many popular salts at SolubilityOFthings. Solubility of nitrogen and nitrogen compounds. The Manufacturing Process Processing rock salt 1 Underground salt deposits are usually discovered by prospectors searching for water or oil. Are the products soluble in water.
Source:
Nitrates and ammonia dissolve. Most test results will be expressed as milligrams per Liter mgL which is the same as parts per million ppm in aquatic solutions. Silver nitrate like most ionic compounds dissolves readily in water. There are certain fertilizers that can be mixed with urea but lose their effectiveness after 2-3 days because of the reactions that occur between the fertilizers chemicals. A magnesium sulfate solution is prepared by mixing 500 g of solid magnesium sulfate in 200 mL of water.
 Source: sciencedirect.com
Source: sciencedirect.com
Nitrates and ammonia dissolve. For very soluble substances like sodium nitrate NaNO 3 this value can be quite high exceeding 100 moles per liter of solution in some cases. Method II Ion. Salt Solubility in cold water glitre Solubility in hot water glitre Sodium chloride 357 391 Potassium chloride 344 567 Calcium chloride 745 1590 Organoleptic properties The taste threshold of the chloride anion in water is dependent on the associated cation. SOLUBILITY PRODUCT CONSTANTS The solubility product constant K sp is a useful parameter for calculating the aqueous solubility of sparingly soluble compounds under various conditions.
 Source: pmstechnoreview.weebly.com
Source: pmstechnoreview.weebly.com
Its solubility in water corresponds to 122g100mL at 0 o C and 256g100mL at a temperature of 25 o. Even the nitrate products are oxidizing agents. These chemicals include magnesium carbonate calcium silicate calcium phosphate magnesium silicate and calcium carbonate. For very soluble substances like sodium nitrate NaNO 3 this value can be quite high exceeding 100 moles per liter of solution in some cases. The crystal structure of AgNO 3 is orthorhombic.
 Source: docbrown.info
Source: docbrown.info
Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. It may be determined by direct measure-ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states. A mixture consisting of barium dioxide barium nitrate magnesium and zinc exploded from an unknown cause demolishing a small plant. Method I Thorium Nitrate Colorimetric Method. For very soluble substances like sodium nitrate NaNO 3 this value can be quite high exceeding 100 moles per liter of solution in some cases.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title magnesium nitrate solubility by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.