Lead nitrate health and reactivity hazard
Home » chemical » Lead nitrate health and reactivity hazard >Lead nitrate health and reactivity hazard
Lead Nitrate Health And Reactivity Hazard. Handling and storage Handling Use only under a chemical fume hood. 29 CFR 1910119 Process Safety Management of Highly Hazardous Chemicals – Compliance Guidelines and Enforcement Procedures NOTE. Lead is a chemical element with the symbol Pb from the Latin plumbum and atomic number 82. OSHA Instruction CPL 2-245A CH-1 September 13 1994.

The same applies to lead compounds such as lead acetate lead oxide lead nitrate and lead carbonate. All CrVI-containing compounds were once thought to be man-made with only CrIII naturally ubiquitous in air water soil and biological materials. Aluminium is also recycled from scrap. This leads to an increase in mobility and availability of uranium to groundwater and soil from nuclear waste repositories which leads to health hazards. Nevertheless plants can take up high levels of lead up to 500 ppm from soils. Lead has the highest atomic number of any stable element.
Bauxite is the most important raw material used in the production of aluminium.
Potential Health Effects Eye. 29 CFR 1910119 Process Safety Management of Highly Hazardous Chemicals – Compliance Guidelines and Enforcement Procedures NOTE. In the United States in the past there was. LeadIIsalts and organic lead compounds are most harmful ecotoxicologically. Chemical Exposure Health Data Chemical Facility Safety and Security Executive Order Chemical Hazards and Toxic Substances Chemical Hazard Assessment Chemical Information Manual see Occupational Chemical Database Chemical Reactivity Hazards Chemicals. Directorate of Compliance Programs.
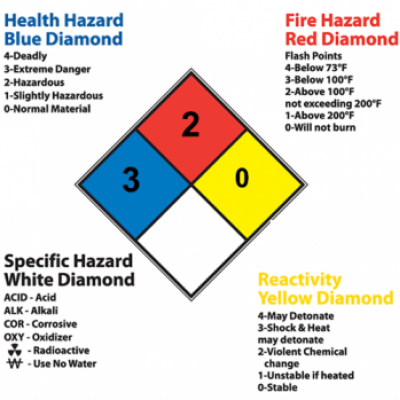 Source: chem.duke.edu
Source: chem.duke.edu
Nitrate is a common pollutant in both natural and engineered water environments. Bauxite is refined to produce alumina from which aluminium metal is recovered by electrolytic reduction. Based on the experimental conditions of a 25 mg dosage of bismuth microspheres and a reaction time of 60 min. Nitrate removal has thus been a long-standing challenge. Acids and alkalis.

See Stability and Reactivity Section 10. Hazard statement means a statement assigned to a hazard class and category that describes the nature of the hazards of a chemical including where appropriate the degree of hazard. Ammonium nitrate and fertilizers containing nitrate particularly when heated. In 2012-2015 that value is 5 micrograms per deciliter µg. LeadIIsalts and organic lead compounds are most harmful ecotoxicologically.
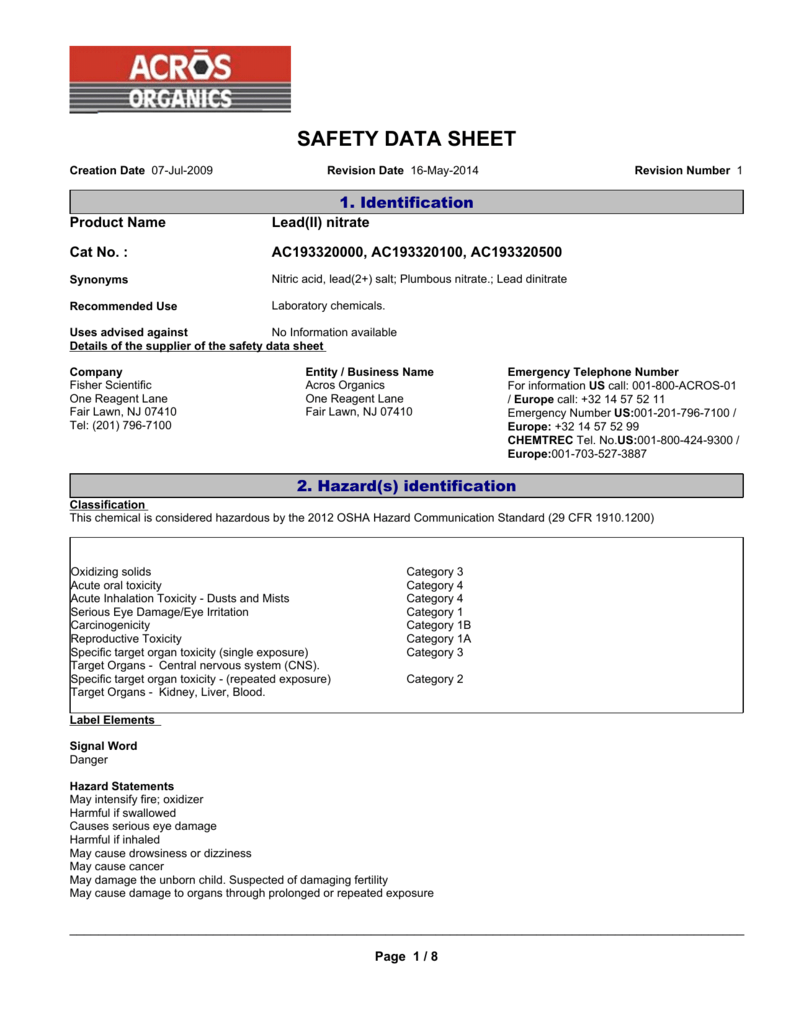 Source: studylib.net
Source: studylib.net
Directorate of Compliance Programs. Use any means of extinction appropriate for the surrounding fire conditions such as water spray carbon dioxide dry chemical or foam. Health 2 Flammability 0 Instability 2 Physical hazards OX _____ Page 3 8 _____ LeadII nitrate Revision Date 19-Jan-2018 Up etc away from spilled material. Federal Regulations SARA 313 Component CAS-No Weight SARA 313 - Threshold Values Ammonium nitrate 6484-52-2 95 10 SARA 311312 Hazard CategoriesSee section 2 for more information CWA Clean Water Act Not applicable Clean Air Act Not applicable OSHA - Occupational Safety and Health. In this study inert bismuth microspheres were developed for the efficient removal of nitrate from water under ultraviolet irradiation.
 Source: mt.com
Source: mt.com
Use any means of extinction appropriate for the surrounding fire conditions such as water spray carbon dioxide dry chemical or foam. The same applies to lead compounds such as lead acetate lead oxide lead nitrate and lead carbonate. Carp Cyprinus carpio were held for 48 hr in lead nitrate concn of between 0 and 20 mg leadL with and without one of the three complexans EDTA NTA or DTPAThe accumulation of lead in both viscera and gills was dose-related with the highest levels for viscera and gills being 86000 and 4560 mgkg dry. Acids and alkalis. Generation rate is greatly increased with smaller particles eg fines and dusts.

Wastes that are hazardous due to the reactivity characteristic may be unstable under normal conditions may react with water may give off toxic gases and may be capable of detonation or explosion under normal conditions or when heated. Diisocyanates are manufactured for the production of polyurethanes a class of polymers. In the United States in the past there was. Ammonium nitrate and fertilizers containing nitrate particularly when heated. In 2004 primary aluminium was being produced.

Wear personal protective equipmentface protection. In the United States in the past there was. This type of hazard often is traced to storage of caustic or toxic cleaning and sanitizing chemicals in food storage containers. Health 2 Flammability 0 Instability 2 Physical hazards OX _____ Page 3 8 _____ LeadII nitrate Revision Date 19-Jan-2018 Up etc away from spilled material. Certain uses lead to the presence of aluminium in drinking water and foodstuffs.
 Source: flinnsci.com
Source: flinnsci.com
Health 2 Flammability 0 Instability 2 Physical hazards OX _____ Page 3 8 _____ LeadII nitrate Revision Date 19-Jan-2018 Up etc away from spilled material. The SDS contains key health and safety information like the chemicals hazardous properties such as its flammability or reactivity or toxicity for example as well as providing information on cleaning up spills providing first aid firefighting and storage incompatibilities. Potential Health Effects Eye. Nitrate is a common pollutant in both natural and engineered water environments. Nitrate removal has thus been a long-standing challenge.

Immediately flush eyes with plenty of water for at least 15 minutes occasionally. This information is used to inform risk management processes. Sweep up and shovel into suitable containers for disposal. Bauxite is the most important raw material used in the production of aluminium. Organic compounds that contain an isocyanate group are referred to as isocyanates.

Chemical Exposure Health Data Chemical Facility Safety and Security Executive Order Chemical Hazards and Toxic Substances Chemical Hazard Assessment Chemical Information Manual see Occupational Chemical Database Chemical Reactivity Hazards Chemicals. The same applies to lead compounds such as lead acetate lead oxide lead nitrate and lead carbonate. Keep away from clothing and other combustible materials. In 2004 primary aluminium was being produced. In nature UVI forms highly soluble carbonate complexes at alkaline pH.
 Source: sciencedirect.com
Source: sciencedirect.com
May cause skin irritation. The toxicity of nitrates is due to their in-vivo conversion to nitrites which may lead to methemoglobinemia. The same applies to lead compounds such as lead acetate lead oxide lead nitrate and lead carbonate. May cause eye irritation. Aluminium hydroxide is produced from bauxite.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lead nitrate health and reactivity hazard by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.