Is sulfur dioxide flammable
Home » chemical » Is sulfur dioxide flammable >Is sulfur dioxide flammable
Is Sulfur Dioxide Flammable. Because it is heavier than air hydrogen sulfide can collect in low-lying and. It is extremely flammable and highly toxic. Trail Operations Trail British Columbia V1R 4L8 Emergency Telephone. Up to 20 ppm.
 What Is Sulfur Dioxide Definition Formula Uses Video Lesson Transcript Study Com From study.com
What Is Sulfur Dioxide Definition Formula Uses Video Lesson Transcript Study Com From study.com
Industrially SO 3 is made by the contact process. Sulfur dioxide is produced by the burning of sulfur or iron pyrite a sulfide ore of iron. The products of the reaction also include small amounts of zinc oxide ZnO and sulfur dioxide SO 2. Airborne dust levels at or above their flammable limits or permissible exposure limits PELs or that prevent visibility of fewer than 5 feet. Copper Acetylene hydrogen peroxide. In the first demonstration below powdered zinc and sulfur.
Oil and gas refining.
Oxygen concentration above 235 percent or below 195 percent. Normal average concentration. Hydrogen sulfide burns to give the gas sulfur dioxide which is more familiar to people as the odor of a burnt match. Pulp and paper processing. Like all alkali metals lithium is highly reactive and flammable and is stored in mineral oil. Water Smells like Rotten Eggs.
 Source: study.com
Source: study.com
Air mixtures with as little as 0000001 H 2 S are associated with a rotten egg smell. Oil and gas refining. Non-flammable Lethal dose or concentration LD LC. In the first demonstration below powdered zinc and sulfur. Chlorine Dioxide is a HIGHLY FLAMMABLE and REACTIVE gas and a DANGEROUS FIRE and EXPLOSION HAZARD.
 Source: icandochemistry.com
Source: icandochemistry.com
Substances which on contact with water emit flammable gases. This reaction produces enough hot gas to propel small rockets. It makes a hugely effective insulating material for medium and high-voltage electrical installations. It is abundant multivalent and nonmetallicUnder normal conditions sulfur atoms form cyclic octatomic molecules with a chemical formula S 8Elemental sulfur is a bright yellow crystalline solid at room temperature. MS H 2 O MO H 2 S.
 Source: firefighterinsider.com
Source: firefighterinsider.com
Up to 20 ppm. Of course do not use any quantity of SF 6 without proper training and precautions. Nitrogen dioxide is a strong oxidizing agent and. It along with aerosols is responsible for the reddish-brown color of smog. Unfortunately however our sense.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is a soft silvery-white alkali metal. Sulphur is a chemical element with the symbol S and atomic number 16. Hydrogen sulfide burns to give the gas sulfur dioxide which is more familiar to people as the odor of a burnt match. Oxygen concentration above 235 percent or below 195 percent. Sulfur is the tenth most common element by mass in the universe.

Nitrogen dioxide is a strong oxidizing agent and. Industrially SO 3 is made by the contact process. DOT regulations FORBID the transport of Chlorine Dioxide in Not Hydrate form. Sulphur is a chemical element with the symbol S and atomic number 16. Cumene Hydroperoxide Acids Cyanides Acids Flammable Liquids Ammonium nitrate chromic acid hydrogen peroxide nitric.
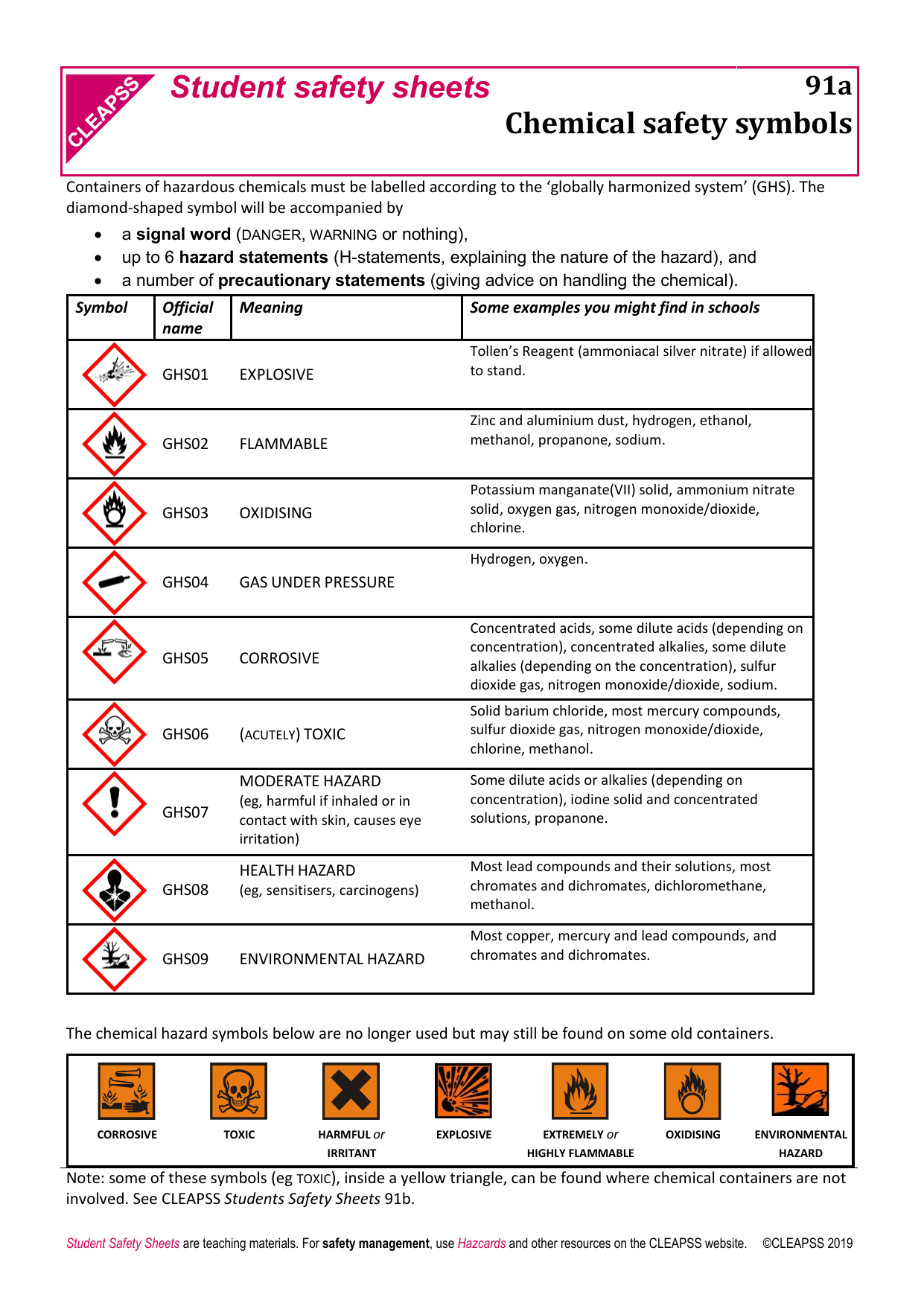 Source: studylib.net
Source: studylib.net
Trade Names and Synonyms. APF Assigned Protection Factor. Air mixtures with as little as 0000001 H 2 S are associated with a rotten egg smell. Up to 20 ppm. Sulfur dioxide is produced by the burning of sulfur or iron pyrite a sulfide ore of iron.
 Source: slideserve.com
Source: slideserve.com
Or Any supplied-air respirator. Like all alkali metals lithium is highly reactive and flammable and is stored in mineral oil. IDENTIFICATION Chlorine Dioxide is a yellow to red gas with an irritating odor similar to Chlorine. Nitrogen dioxide is a strong oxidizing agent and. The products of the reaction also include small amounts of zinc oxide ZnO and sulfur dioxide SO 2.
 Source: studocu.com
Source: studocu.com
Sulfur is the tenth most common element by mass in the universe. This was one of the model rocket propellants described by Homer Hickam in his book Rocket Boys Delacorte Pr. Industrially SO 3 is made by the contact process. Hydrogen sulfide burns to give the gas sulfur dioxide which is more familiar to people as the odor of a burnt match. SO 2 1 2 O 2 SO 3 ΔH-1984 Industrial.

Small amounts of hydrogen sulfide occur in crude petroleium but natural gas can contain up to 28. Hydrogen sulfide also occurs naturally in sewers manure pits well water oil and gas wells and volcanoes. Sulphur is a chemical element with the symbol S and atomic number 16. Small amounts of hydrogen sulfide occur in crude petroleium but natural gas can contain up to 28. Trade Names and Synonyms.
 Source: calitateaer.ro
Source: calitateaer.ro
Substances liable to spontaneous combustion. Air mixtures with as little as 0000001 H 2 S are associated with a rotten egg smell. Flammable solids self-reactive substances solid desensitised explosives and polymerizing substances. Sulfur reacts with Group I metal nitrides to form flammable mixtures evolving flammable and toxic NH3 and H2S gases if water is present Mellor 1940 Vol. A person trained by an expert can breathe a small quantity of sulfur hexafluoride without harm to demonstrate how sound travels through a very dense gas.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is sulfur dioxide flammable by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.