Is ammonium chloride an acid
Home » chemical » Is ammonium chloride an acid >Is ammonium chloride an acid
Is Ammonium Chloride An Acid. 84 Protein Precipitation by Means of Ammonium Sulfate. An example of an acid salt is one containing any of these cations with a neutral base such as ammonium chloride NH 4 Cl. For example the ammonium ion is the conjugate acid of ammonia a weak base. Draize test rabbit eye.
 Title Lesson 10 Salt Hydrolysis Ppt Video Online Download From slideplayer.com
Title Lesson 10 Salt Hydrolysis Ppt Video Online Download From slideplayer.com
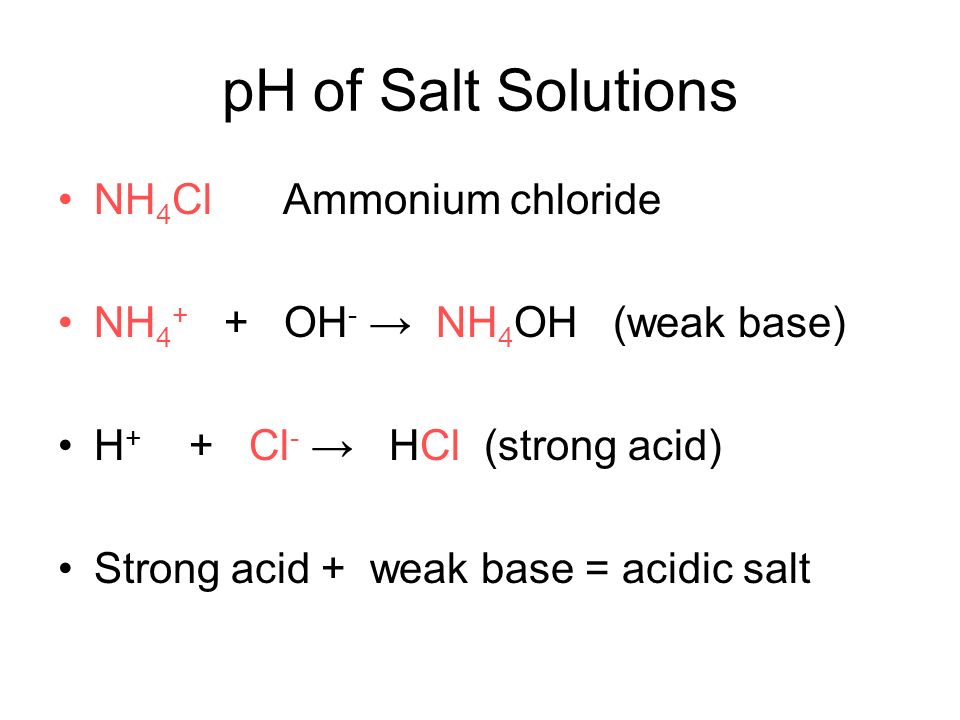
58 Monday March 26 2012 Rules and Regulations Issue date. Check the chemical compatibility of Polyvinyl chloride PVC with various chemicals solvents alcohols and other products. Ammonia is a Bronsted-Lowry base. Typically quats do not exhibit efficacy against difficult-to-kill nonenveloped viruses such as norovirus rotavirus or poliovirus. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. An example of an acid salt is one containing any of these cations with a neutral base such as ammonium chloride NH 4 Cl.
Organic Acids Acetic acid Butyric acid n- Formic acid Propionic acid Rosin Oil Tall oil Group 3.
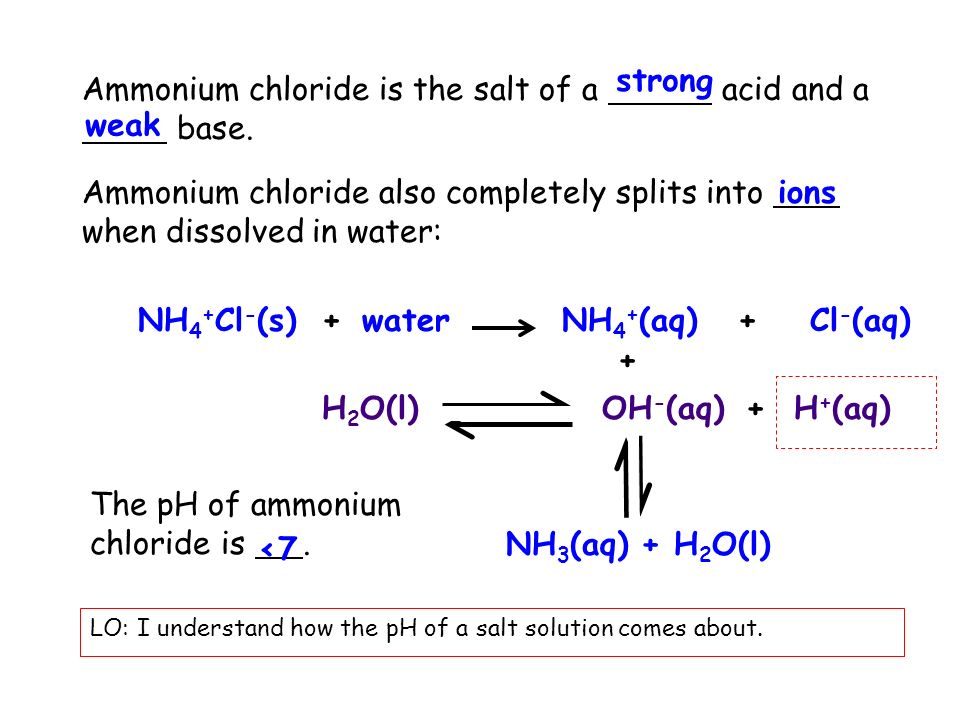
NH 4 Cls – NH 4 aq Cl-aq NH 4 H 2 Ol – NH 3 aq H 3 O aq. Caustics Caustic potash solution Caustic soda solution Group 4. Section 11 - Toxicological Information RTECS. This acid-forming salt also has an expectorant action by irritating the mucous membranes making it useful for cough relief. By far the largest amount of sulfuric acid is used to make phosphoric acid used in turn to make the phosphate fertilizers calcium dihydrogenphosphate and the ammonium phosphates. Hydrochloric acid concentration in the stomach is approximately 05 percent or 5000 parts per million.
Amines and Alkanolamines Aminoethylethanolamine Aniline Diethanolamine. Amines and Alkanolamines Aminoethylethanolamine Aniline Diethanolamine. Hydrofluoric acid aqueous Hydrogen chloride anhydrous Hydrogen fluoride anhydrous Nitric acid Oleum Phosphoric acid Sulfuric acid Group 2. The compound is a cationic surfactant and cations generally adsorb more strongly to. Section 11 - Toxicological Information RTECS.
 Source: youtube.com
Source: youtube.com
Einige Bemerkungen über den Begriff der Säuren und Basen Some. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. In patients with normal hepatic function the ammonium cation is converted to urea by the liver and a hydrogen cation is released which reacts with a bicarbonate ion to form water and carbon dioxide. Ammonium Chloride Safety Data Sheet according to Federal Register Vol. To 352mg of alumi- of a freshly prepared mixture of 015mL of sodium.
 Source: youtube.com
Source: youtube.com
Also the presence of ionic surfactants especially cationic surfactants is a reason. It is also found around some types of volcanic vents. Typically quats do not exhibit efficacy against difficult-to-kill nonenveloped viruses such as norovirus rotavirus or poliovirus. Ammonium Chloride Safety Data Sheet according to Federal Register Vol. The chloride anion combines with fixed bases in the extracellular.
 Source: topblogtenz.com
Source: topblogtenz.com
Protein precipitation prior to CPLL treatment is justified when a protein extract contains molecules incompatible with a proper capture. This low stomach fluid level normally leaves it free from microbes. By far the largest amount of sulfuric acid is used to make phosphoric acid used in turn to make the phosphate fertilizers calcium dihydrogenphosphate and the ammonium phosphates. Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. 10 mgm3 TWA.
 Source: slideplayer.com
Source: slideplayer.com
5g of Sodium Chloride and dilute with water to 250mL. The acid-forming properties of ammonium chloride result from dissociation of the salt to an ammonium cation and a chloride anion. This low stomach fluid level normally leaves it free from microbes. Substance Substance name. 10 mgm3 TWA.
 Source: slideplayer.com
Source: slideplayer.com
12125-02-9 Product code. Organic Acids Acetic acid Butyric acid n- Formic acid Propionic acid Rosin Oil Tall oil Group 3. Hydrofluoric acid aqueous Hydrogen chloride anhydrous Hydrogen fluoride anhydrous Nitric acid Oleum Phosphoric acid Sulfuric acid Group 2. Ammonium Chloride Safety Data Sheet according to Federal Register Vol. Caustics Caustic potash solution Caustic soda solution Group 4.

Muby Chemicals of Mubychem Group established in 1976 is the original manufacturers of Specialty Chemicals Pharmaceutical Excipient Fragrance Food Flavor chemicals Reagent Grade Chemicals Shale Gas Fracturing Chemicals in India. 84 Protein Precipitation by Means of Ammonium Sulfate. A balanced stomach s pH is generally 10-20. 10 mgm3 TWA. Hydrochloric acid is a Bronsted-Lowry acid.
 Source: slideplayer.com
Source: slideplayer.com
The cation is the conjugate acid of a weak base. Its conjugate acid is the ammonium ion. Some concentrated formulations have been shown to be effective low-level disinfectants. In this case both of the reactants are salts. When you get questions like this it helps a lot if you can see the general sorts of patterns.
 Source: freechemistryonline.com
Source: freechemistryonline.com
12125-02-9 Product code. The acid-forming properties of ammonium chloride result from dissociation of the salt to an ammonium cation and a chloride anion. Amines and Alkanolamines Aminoethylethanolamine Aniline Diethanolamine. Also the presence of ionic surfactants especially cationic surfactants is a reason. I will give the lowest level of explanation and then follow up with a high level explanation.
 Source: youtube.com
Source: youtube.com
Also the presence of ionic surfactants especially cationic surfactants is a reason. Uses of sulfuric acid. A balanced stomach s pH is generally 10-20. Solutions of ammonium chloride are mildly acidic. Bisulfate HSO 4 dihydrogen citrate H 2 C 6 H 5 O 7 bioxalate HO 2 C 2 O Each of these anions.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is ammonium chloride an acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.