Hooc mass number
Home » chemical » Hooc mass number >Hooc mass number
Hooc Mass Number. Materials that change phase eg via melting can store thermal energy with energy densities comparable to batteries. Therefore the atom economy 100 x 112 244 459. So the molarity for OH- would be 000002501 000025 molL If we take the negative 10log of that we get-10log000025 pOH 36. Nhoocch24cooh nh2nch26 nh2 553k -n-ch2 6-n-.
 Cyclic Voltammetry Curves Of A Hooc Ani 4 Cooh B Hooc Ani Download Scientific Diagram From researchgate.net
Cyclic Voltammetry Curves Of A Hooc Ani 4 Cooh B Hooc Ani Download Scientific Diagram From researchgate.net
Out of a total mass of reactants or products of 160 84 112 132 244. C 10 H 18 O 4 or HOOCCH 2 8 COOH. The molar mass of NaOH is about 40 gmol. One mole of any substance has a mass equal to the relative molecular mass RMM of that substance in grams. Another form of condensation polymerization is the reaction between a dibasic acid and a glycol where the polymer formed is known as polyester. Test your Knowledge on Condensation polymerization.
Sublimes slowly at 750 mm Hg when heated to melting point.
Molarity refers to the number of moles of the solute present in 1 liter of solution. When glutamic acid is dissolved in water the amino group NH 2 may gain a proton H andor the carboxyl groups may lose protons depending on the acidity of the medium. Would I drink that. So the molarity for OH- would be 000002501 000025 molL If we take the negative 10log of that we get-10log000025 pOH 36. Immediate steps should be taken to limit its spread to the. For example 1 mole of H2SO4 is equal to 9808 grams of H2SO4 molecular weight 9808.
Source: chemspider.com
Nylon-66 is obtained by the polymerisation of adipic acid with hexamethylene diamine. Visit BYJUS to understand the properties structure and uses of Oxalic acid C2H2O4 explained by Indias best teachers. Oxalic acid COOH2 - Oxalic acid is the smallest di-carboxylic acid with the chemical formula C2H2O4. In simple words 1 mole is equal to the atomic weight of the substance. I State the name given to atoms of the same element with different nucleon numbers.
 Source: researchgate.net
Source: researchgate.net
Nylon-66 is obtained by the polymerisation of adipic acid with hexamethylene diamine. Therefore it is useful to have summaries of phase change properties over a wide range of materials. Predict the major product of the following reaction. Academiaedu is a platform for academics to share research papers. For ethanol C 2H 5OH RMM 2 x 12010 g mol-1 6 x 1006 g mol-1 15999 g mol-1 46057 g mol-1 0152 g of an organic compound X.

Test your Knowledge on Condensation polymerization. In sufficiently acidic environments the amino group gains a proton and the molecule becomes a cation with a single positive charge HOOCCHNH 3CH 2 2 COOH. Molar mass of acetic acid CH3COOH 6005 gmole. Therefore it is useful to have summaries of phase change properties over a wide range of materials. Concentration of Sulfuric acid.
 Source: rapidonline.com
Source: rapidonline.com
Immediate steps should be taken to limit its spread to the. Empirical formula calculator for ionic compounds. It is insoluble in water. Slightly soluble in water. Molarity refers to the number of moles of the solute present in 1 liter of solution.
 Source: doubtnut.app
Source: doubtnut.app
HOOCCH 2 n COOH HOCH 2 m OH HOOC CH 2 n COOCH 2 m OH H 2 O. Nylon-66 is obtained by the polymerisation of adipic acid with hexamethylene diamine. Molarity refers to the number of moles of the solute present in 1 liter of solution. Would I drink that. 3 LE 2021 06204221 Turn over 2 Silver has an atomic number of 47.
 Source: youtube.com
Source: youtube.com
Out of a total mass of reactants or products of 160 84 112 132 244. Element number 104 in the Periodic Table is A an s-block element B a p-block element C a d-block element D an f-block element. Molecular weight of H2SO4. As pHpOH 14 this gives an pH of 104 That is strongly basic. For example they induce apoptosis of proinflammatory T cells suppress B cell antibody production and reduce neutrophil migration during inflammation.

H H O NH3 H N H2 H N Ni 18. Slightly soluble in water. Element number 104 in the Periodic Table is A an s-block element B a p-block element C a d-block element D an f-block element. Sublimes slowly at 750 mm Hg when heated to melting point. A Raoults Law b Henrys Law c Nernsts Distribution Law d None of these 7.
 Source: researchgate.net
Source: researchgate.net
Relative molecular mass is the sum of the relative atomic masses RAMs of the constituent elements in the compound. These are the condensation polymers of diamines and dibasic acids a number is usually suffixed with the nylon which refers to the number of carbon atoms present in the diamine and the dibasic acids respectively. H H O NH3 H N H2 H N Ni 18. It doesnt matter whether you use the total mass of reactants or the total mass products in the calculations they are the same due to the law of conservation of mass. At pH values between about 25 and 41 the.
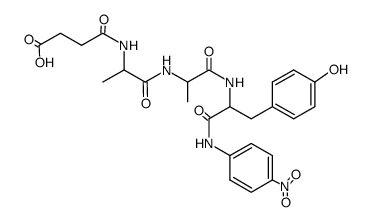 Source: chemsrc.com
Source: chemsrc.com
Predict the major product of the following reaction. The primary hazard is the threat to the environment. Density of glacial acetic acid. A Proportional to the molarity of the. Concentration of Sulfuric acid.

3 LE 2021 06204221 Turn over 2 Silver has an atomic number of 47. These are the condensation polymers of diamines and dibasic acids a number is usually suffixed with the nylon which refers to the number of carbon atoms present in the diamine and the dibasic acids respectively. A Raoults Law b Henrys Law c Nernsts Distribution Law d None of these 7. Element number 104 in the Periodic Table is A an s-block element B a p-block element C a d-block element D an f-block element. Sebacic acid is a white granular powder.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hooc mass number by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.