Hazards of sodium bicarbonate
Home » chemical » Hazards of sodium bicarbonate >Hazards of sodium bicarbonate
Hazards Of Sodium Bicarbonate. Generally elemental sodium is more reactive than lithium and it reacts with water to form a strong base sodium hydroxide NaOH. The Meaning of pH. When heated to decomposition this compound emits toxic fumes of carbon monoxide carbon dioxide and sodium oxides. Sodium bicarbonate is then heated to give Na 2 CO 3.
Dept Harpercollege Edu From
Intratumor injections such as TILA-TACE are quite more difficult to perform compared with oral delivery. And in patients receiving corticosteroids or corticotropin since each gram of sodium bicarbonate contains about 12 mEq of sodium. Sodium bicarbonate should be used with extreme caution in patients with congestive heart failure or other edematous or sodium-retaining conditions. When heated to decomposition this compound emits toxic fumes of carbon monoxide carbon dioxide and sodium oxides. NTP 1992 Reactivity Profile. In patients with renal insufficiency especially those with severe insufficiency such as oliguria or anuria.
Molecular Weight Molar Mass.
Various Please Contact Supplier for Further Information Supplier. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air. The packaging indicates that each Alka Seltzer tablet contains 325 mg of aspirin acetylsalicylic acid 1000 mg of citric acid and 1916 mg of sodium bicarbonate. Anhydrous sodium carbonate is dissolved in water and recrystallizes to get washing soda crystals containing 10 molecules of water of crystallization. Here students are encouraged to determine the percent by mass of sodium bicarbonate in an Alka Seltzer tablet by carrying out this simple acidbase reaction. The corrosion of solid sodium by oxygen also is.
 Source: yumpu.com
Source: yumpu.com
Anhydrous sodium carbonate is dissolved in water and recrystallizes to get washing soda crystals containing 10 molecules of water of crystallization. Here students are encouraged to determine the percent by mass of sodium bicarbonate in an Alka Seltzer tablet by carrying out this simple acidbase reaction. Pure water has a neutral pH value of 7. The CO 2 gas evolved can be reused again. Baking soda also known as sodium bicarbonate has a pH of 9 making it a mildly alkaline substance.
 Source: studylib.net
Source: studylib.net
Sodium Bicarbonate Chemical name. Stronger acids and alkalis have. Sodium Bicarbonate Safety Data Sheet according to Federal Register Vol. When heated to decomposition this compound emits toxic fumes of carbon monoxide carbon dioxide and sodium oxides. Pure water has a neutral pH value of 7.
Source:
The packaging indicates that each Alka Seltzer tablet contains 325 mg of aspirin acetylsalicylic acid 1000 mg of citric acid and 1916 mg of sodium bicarbonate. The CO 2 gas evolved can be reused again. Generally elemental sodium is more reactive than lithium and it reacts with water to form a strong base sodium hydroxide NaOH. Sodium hydrogencarbonate commonly known as baking soda or bicarbonate of soda is a chemical compound with the formula NaHCO 3It is a salt composed of a sodium cation Na and a bicarbonate anion HCO 3 Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powderIt has a slightly salty alkaline taste resembling that of. 12 09132019 EN English US Page 1 SECTION 1.
 Source: yumpu.com
Source: yumpu.com
Sodium Bisulfite Solution Manufacturer. Various Please Contact Supplier for Further Information Supplier. Sodium Bicarbonate Chemical name. Sodium bicarbonate that reacted can be calculated using the weight loss. The corrosion of solid sodium by oxygen also is.
 Source: yumpu.com
Source: yumpu.com
Sodium hydrogencarbonate commonly known as baking soda or bicarbonate of soda is a chemical compound with the formula NaHCO 3It is a salt composed of a sodium cation Na and a bicarbonate anion HCO 3 Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powderIt has a slightly salty alkaline taste resembling that of. Additional sodium bicarbonate may be required for use in incubators containing higher percentages of CO 2. Intratumor injections such as TILA-TACE are quite more difficult to perform compared with oral delivery. Sodium bicarbonate IUPAC name. The scale ranges from -1 to 15 with low values being acidic and high values being alkaline.
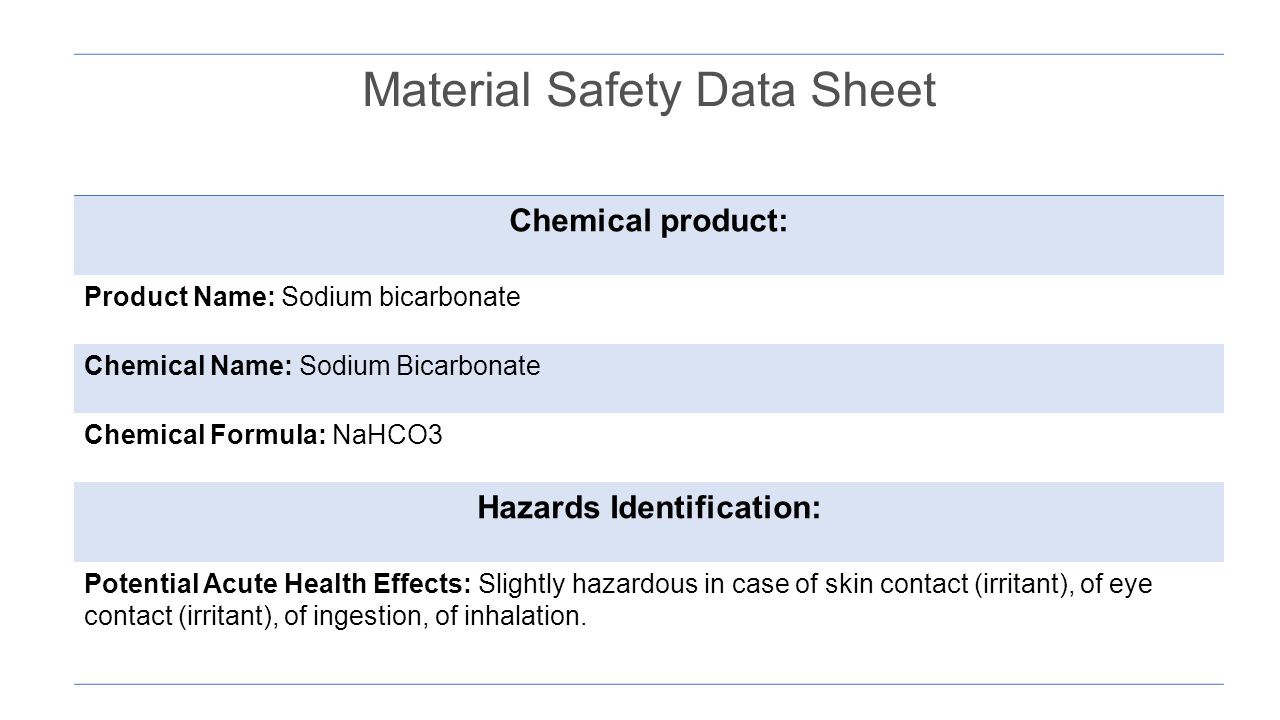 Source: slideplayer.com
Source: slideplayer.com
PRODUCT AND COMPANY IDENTIFICATION Product Identity. Sodium Chloride is a metal halide composed of sodium and chloride with sodium and chloride replacement capabilities. Baking soda also known as sodium bicarbonate has a pH of 9 making it a mildly alkaline substance. Stronger acids and alkalis have. PRODUCT AND COMPANY IDENTIFICATION Product Identity.
Source:
Properties of Sodium Carbonate Na 2 CO 3. NTP 1992 Reactivity Profile. The pH of a substance is a measure of acidity and alkalinity. Pure water has a neutral pH value of 7. The Meaning of pH.
 Source: yumpu.com
Source: yumpu.com
The CO 2 gas evolved can be reused again. 12 09132019 EN English US Page 1 SECTION 1. PRODUCT AND COMPANY IDENTIFICATION Product Identity. The CO 2 gas evolved can be reused again. Disodium pyrophosphate or sodium acid pyrophosphate SAPP is an inorganic compound consisting of sodium cations and pyrophosphate anion.
Source:
Molecular Weight Molar Mass. Sodium bicarbonate should be used with extreme caution in patients with congestive heart failure or other edematous or sodium-retaining conditions. Na 2 CO 3. Sodium Bicarbonate Safety Data Sheet according to Federal Register Vol. Pure water has a neutral pH value of 7.
 Source: coursehero.com
Source: coursehero.com
Here students are encouraged to determine the percent by mass of sodium bicarbonate in an Alka Seltzer tablet by carrying out this simple acidbase reaction. Sodium Bisulfite Solution Manufacturer. When heated to decomposition this compound emits toxic fumes of carbon monoxide carbon dioxide and sodium oxides. Substance Substance name. PRODUCT AND COMPANY IDENTIFICATION Product Identity.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hazards of sodium bicarbonate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.