Hazards of naoh
Home » chemical » Hazards of naoh >Hazards of naoh
Hazards Of Naoh. It is a white crystalline solid with a sulfurous Salty taste. 수산화 나트륨水酸化 화학식. And temporary loss of hair. NaOH은 물에 녹아 강염기성 수용액을 만든다.
 Hns Ms From hns-ms.eu
Hns Ms From hns-ms.eu
Sodium hydroxide NaOH or HNaO CID 14798 - structure chemical names physical and chemical properties classification patents literature biological activities. A lye is a metal hydroxide traditionally obtained by leaching wood ashes or a strong alkali which is highly soluble in water producing caustic basic solutions. Solid NaOH has a melting point and boiling point of approximately 604F and 2534F. NaOH Sodium hydroxide 50 1310-73-2. It can cause irritation to the eyes skin and mucous membrane. Sodium hydroxide is very corrosive.
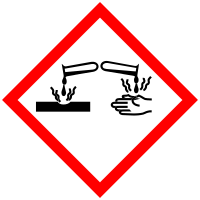
It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH.
Eye and skin burns. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Freeman NY 2003 p. It is a white crystalline solid with a sulfurous Salty taste. Potassium hydroxide is also a precursor to other potassium compounds.
 Source: relatingchemistry.blogspot.com
Source: relatingchemistry.blogspot.com
Dung dịch NaOH có tính nhờn làm bục vải giấy và ăn mòn da. Always wear gloves when handling NaOH-treated filters due to their caustic nature. Natri hydroxide hay hydroxide natri công thức hóa học. It also contains information about other experiments that often occur in A-level examinations. G271 - Auditing health safety in a secondary school science department.
 Source: mysafetylabels.com
Source: mysafetylabels.com
Hazardous goods shipment required both from the SLTC to the field and from the field to the SLTC. 화학 실험에서 가장 널리 사용되는 염기이며 산업계에서는 흔히 가성소다라고 일컫는다. Hazardous goods shipment required both from the SLTC to the field and from the field to the SLTC. Natri hydroxide hay hydroxide natri công thức hóa học. Lye most commonly refers to sodium hydroxide NaOH but historically has been used for potassium hydroxide KOH.
 Source: websites.umich.edu
Source: websites.umich.edu
You may be asked about other unfamiliar experiments but these will be. Today lye is commercially manufactured using a membrane cell chloralkali processIt is supplied in various forms. Click here to access the journal. Liquid 480 480 480 480 480 480 480 480 480 480. Use clean polytetrafluoroethylene PTFE-coated eg Teflon-coated or plastic tweezers.
 Source: seton.com
Source: seton.com
수산화 나트륨水酸化 화학식. 1998년에 세계적으로 4500만톤이 생산되었다. Safety and hazards Practical Guide AQA This guide includes details about the required practicals for A-level chemistry. The Hazard fields include special hazard alerts air and water reactions fire hazards health hazards a reactivity profile and. May cause chemical conjunctivitis and corneal damage.

Sodium hydroxide 50 1310-73. PVC or nitrile gloves are suggested based on review of chemical resistance data. Eyes skin mucous membranes. As a solid sodium hydroxide is a hygroscopic material and will readily absorb moisture and carbon dioxide from its environment as well as the oils found on human skin. GL238 -Top twenty hazards in science.
 Source: yumpu.com
Source: yumpu.com
Lye most commonly refers to sodium hydroxide NaOH but historically has been used for potassium hydroxide KOH. And temporary loss of hair. Do not use metal tweezers to handle the filters as they will deposit CrVI onto filters. Click here to access the journal. Safety and hazards Practical Guide AQA This guide includes details about the required practicals for A-level chemistry.
 Source: shutterstock.com
Source: shutterstock.com
Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Corrosive Liquid Toxic UN 2922 Class 8 PGIII. Causes digestive and respiratory tract burns. Sodium hydroxide NaOH or HNaO CID 14798 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
 Source: hns-ms.eu
Source: hns-ms.eu
You may be asked to describe these experiments in details or be asked about reasons for doing individual steps. Potassium hydroxide is also a precursor to other potassium compounds. Corrosive Liquid Toxic UN 2922 Class 8 PGIII. It can cause irritation to the eyes skin and mucous membrane. The Hazard fields include special hazard alerts air and water reactions fire hazards health hazards a reactivity profile and.
 Source: pdf4pro.com
Source: pdf4pro.com
Sodium hydroxide NaOH or HNaO CID 14798 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. 화학 실험에서 가장 널리 사용되는 염기이며 산업계에서는 흔히 가성소다라고 일컫는다. A lye is a metal hydroxide traditionally obtained by leaching wood ashes or a strong alkali which is highly soluble in water producing caustic basic solutions. Sodium Sulfite Na2SO3-Sodium Sulfite is an Ionic Salt with the Chemical Formula Na2SO3.

As a solid sodium hydroxide is a hygroscopic material and will readily absorb moisture and carbon dioxide from its environment as well as the oils found on human skin. 식음료 비누 등의 산업에서 널리 사용된다. To learn more about the Structure of Sodium Sulfite Molecules along with the Preparation Properties Health hazards Uses and FAQs Visit BYJUS for complete information. Sodium hydroxide 50 1310-73. Find out more about the ASE and the.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hazards of naoh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.