Hazards of anhydrous sodium sulfate
Home » chemical » Hazards of anhydrous sodium sulfate >Hazards of anhydrous sodium sulfate
Hazards Of Anhydrous Sodium Sulfate. It also helps in the removal of splinters and is quite effective. Substance Substance name. 11 04112018 EN English US Page 1 SECTION 1. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base.
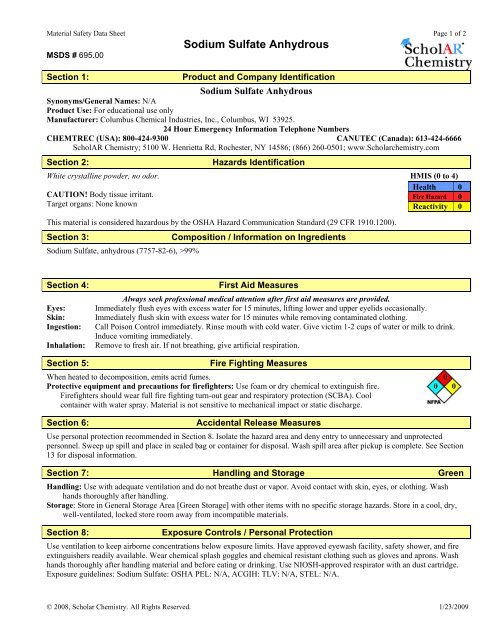
 Material Safety Data Sheet Sodium Sulfate Powder Anhydrous From studylib.net
Material Safety Data Sheet Sodium Sulfate Powder Anhydrous From studylib.net
Sodium sulfate anhydrous is an electrolyte replenisher and is used in isosmotic solutions. In a sodium sulfite molecule there. In its anhydrous form image provided above sodium sulfite is a white solid. Na 2 SO 37H 2 O is slowly oxidized by atmospheric oxygen giving rise to the corresponding sulfate. Sodium hydroxide is also the most common base used in chemical laboratories. 58 Monday March 26 2012 Rules and Regulations Date of issue.
The primary difference between anhydrous Na 2 SO 3 and its heptahydrate is the relative stability of the anhydrous form towards oxidation.
2633 gcm 3 anhydrous. It is commonly referred to as Epsom salt. Identification of the substancemixture and of the supplier Product name. Sodium sulfite sodium sulphite is the inorganic compound with the chemical formula Na 2 SO 3. Identification Product form. Sodium SulfateAnhydrous Created by Global Safety Management Inc.
 Source: studylib.net
Source: studylib.net
It is used to place cobalt into other chemical compounds. Sodium – reacts vigorously with gaseous hydrochloric acid. Sodium hydroxide is also the most common base used in chemical laboratories. It is mainly used as a filler in the manufacture of powdered home laundry. The anhydrous without water blue form can be made by reacting cobalt with chlorine.

In a sodium sulfite molecule there. Na 2 SO 37H 2 O is slowly oxidized by atmospheric oxygen giving rise to the corresponding sulfate. It also helps in the removal of splinters and is quite effective. Identification Product form. The anhydrous without water blue form can be made by reacting cobalt with chlorine.
 Source: studylib.net
Source: studylib.net
Sodium ion is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Sodium Sulfate Anhydrous is the anhydrous sodium salt form of sulfuric acidSodium sulfate anhydrous disassociates in water to provide sodium ions and sulfate ions. It is majorly used as a bath soak to get rid of sore muscles to ease the pain of sprains and bruises etc. Sodium hydroxide is also the most common base used in chemical laboratories.
 Source: yumpu.com
Source: yumpu.com
It is majorly used as a bath soak to get rid of sore muscles to ease the pain of sprains and bruises etc. Acetic anhydride 2-aminoethanol ammonium hydroxide chlorosulfonic acid ethylene diamine ethyleneimine oleum propiolactone sodium hydroxide sulfuric acid and vinyl acetate – increase in temperature and. Sodium sulfite can be prepared by treating a solution. An alkali caustic soda is widely used in many industries mostly as a strong chemical base in the manufacture of pulp and paper textiles drinking water and detergents. A white water-soluble solid it is used commercially as an antioxidant and preservative.
 Source: yumpu.com
Source: yumpu.com
Sodium Sulfate Anhydrous is the anhydrous sodium salt form of sulfuric acidSodium sulfate anhydrous disassociates in water to provide sodium ions and sulfate ions. The following materials should be avoided. It is commonly referred to as Epsom salt. Sodium sulfate anhydrous is an electrolyte replenisher and is used in isosmotic solutions. The anhydrous without water blue form can be made by reacting cobalt with chlorine.

Sodium sulfate also known as sodium sulphate or sulfate of soda is the inorganic compound with formula Na 2 SO 4 as well as several related hydratesAll forms are white solids that are highly soluble in water. Sodium hydroxide is also the most common base used in chemical laboratories. In its anhydrous form image provided above sodium sulfite is a white solid. 58 Monday March 26 2012 Rules and Regulations Date of issue. Magnesium sulfate is a crystalline solid that has a white appearance and is odorless.

Identification Product form. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment. 58 Monday March 26 2012 Rules and Regulations Date of issue. Sodium ion is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. 2633 gcm 3 anhydrous.
 Source: yumpu.com
Source: yumpu.com
Sodium sulfite can be prepared by treating a solution. Acetic anhydride 2-aminoethanol ammonium hydroxide chlorosulfonic acid ethylene diamine ethyleneimine oleum propiolactone sodium hydroxide sulfuric acid and vinyl acetate – increase in temperature and. Identification of the substancemixture and of the supplier Product name. Mercuric sulfate – violent reaction with gaseous hydrochloric acid at 250F. Magnesium sulfate is a crystalline solid that has a white appearance and is odorless.

With an annual production of 6 million tonnes the decahydrate is a major commodity chemical product. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Sodium Sulfate Anhydrous CAS-No. It is used to place cobalt into other chemical compounds.

Acetic anhydride 2-aminoethanol ammonium hydroxide chlorosulfonic acid ethylene diamine ethyleneimine oleum propiolactone sodium hydroxide sulfuric acid and vinyl acetate – increase in temperature and. 11 04112018 EN English US Page 1 SECTION 1. Identification Product form. Structure of Sodium Sulfite- Na 2 SO 3. The following materials should be avoided.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hazards of anhydrous sodium sulfate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.