Ferric iron hazards
Home » chemical » Ferric iron hazards >Ferric iron hazards
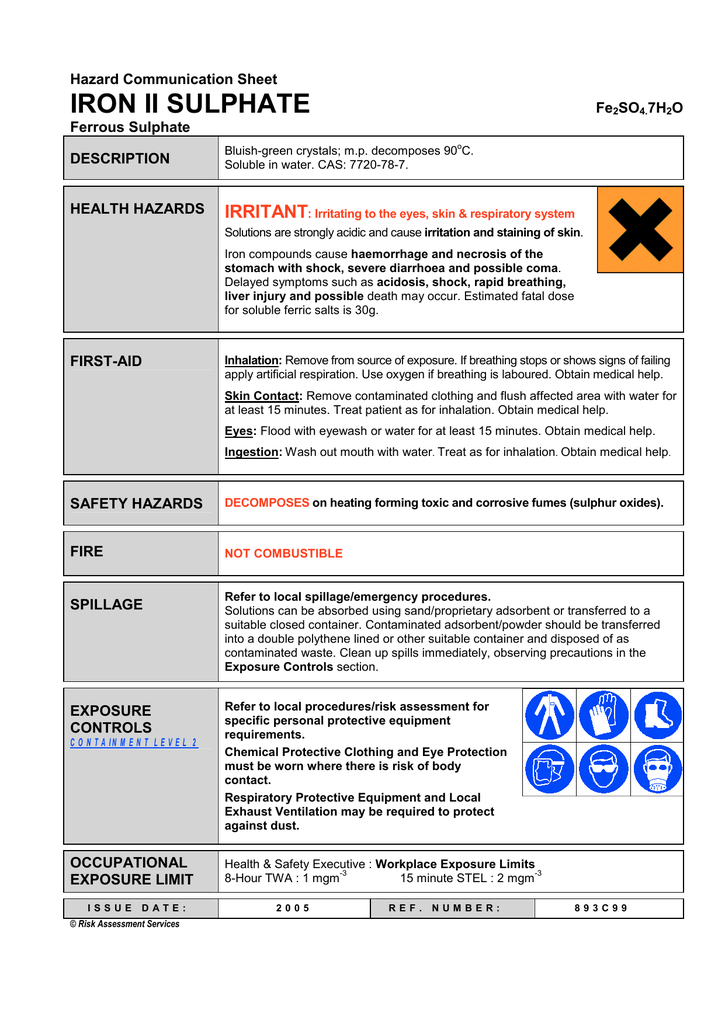
Ferric Iron Hazards. Not classified Ferric Chloride Hexahydrate 10025 -77 -1. 1309-37-1 or 1332-37-2 einecs number. These clinical studies examined IDA in postpartum patients patients with heavy. 5 mgm p 3 iron oxide fume.
 Inhibition Of Perchlorate Biodegradation By Ferric And Ferrous Iron Sciencedirect From sciencedirect.com
Inhibition Of Perchlorate Biodegradation By Ferric And Ferrous Iron Sciencedirect From sciencedirect.com
1309-37-1 or 1332-37-2 einecs number. AC436810250 CAS-No 7782-61-8 Synonyms Ferric nitrate Recommended Use Laboratory chemicals. 5 mgm p 3 iron oxide fume. Typically a piece of iron can take days weeks months and sometimes even years to get that first coat of. By reflected light the crystals appear dark green but by transmitted light they appear purple-red. The removal trends of Cu and Fe were found similar.
001-800-ACROS-01 Europe call.
Uingredient name u ucas number u upercent u uexposure limits u iron oxide red 1309-37-1 92 acgih tlv. Details of the supplier of the safety data sheet Emergency Telephone Number For information US call. Ferric chloride is an orange to brown-black solid. Ferric oxide chemical formula. Pigment red 10177491 cas number. Uingredient name u ucas number u upercent u uexposure limits u iron oxide red 1309-37-1 92 acgih tlv.

When its left exposed to these two it tends to develop a flaky red-brown coat. We know this coat as rust but its full chemical formula is ironIII oxide or Fe²O³. Red iron oxide chemical name. Typically a piece of iron can take days weeks months and sometimes even years to get that first coat of. Health 0 flammability 0 reactivity 0 section ii composition.

5 mgm p 3 iron oxide fume. 001-800-ACROS-01 Europe call. Ferric Iron is clear well water that has been exposed to oxygen oxidized forming visible rust giving the water a reddish color. Ferric oxide chemical formula. Ferric chloride is an orange to brown-black solid.
 Source: ackdjournal.org
Source: ackdjournal.org
1309-37-1 or 1332-37-2 einecs number. Red iron oxide chemical name. Prussian blue C18Fe7N18 CID 2724251 - structure chemical names physical and chemical properties classification patents literature biological activities. Thermal decomposition generates. Typically a piece of iron can take days weeks months and sometimes even years to get that first coat of.
 Source: sciencedirect.com
Source: sciencedirect.com
When its left exposed to these two it tends to develop a flaky red-brown coat. These clinical studies examined IDA in postpartum patients patients with heavy. It is slightly soluble in water. For each subject the hemoglobin incorporation method was used to measure the true absorption of 60 mg of iron from either ferrous sulfate or ferric ammonium citrateThe iron tolerance test ITT was also studied for these two compounds and for reduced iron. Details of the supplier of the safety data sheet Emergency Telephone Number For information US call.
 Source: studylib.net
Source: studylib.net
Substitutes include other aluminum and iron salts like sodium aluminate and ferric sulfate but these may or may not work. There is no set time frame to rusting. Iron and its related alloys react to oxygen and water in a specific way. Red iron oxide chemical name. The color depends on the viewing angle.

For example a few products from ATS Innovas ATS 800 line are excellent substitutes for alum and ferric chloride. Ferric EDTA may serve as plant available iron supplement for the Hoagland solution or the Long Ashton solution. Skin and eye contact Acute toxicity. Red iron oxide chemical name. The solubility constant of K sp for ferric hydroxide and copper hydroxide are 40.
 Source: researchgate.net
Source: researchgate.net
By reflected light the crystals appear dark green but by transmitted light they appear purple-red. 001-800-ACROS-01 Europe call. The removal trends of Cu and Fe were found similar. By reflected light the crystals appear dark green but by transmitted light they appear purple-red. It is slightly soluble in water.
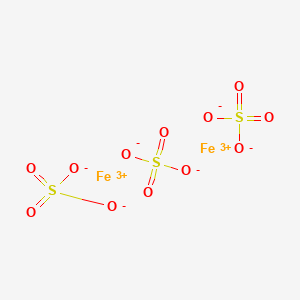
When its left exposed to these two it tends to develop a flaky red-brown coat. Iron and its related alloys react to oxygen and water in a specific way. Pick up and remove spilled solid before adding water. IronIII chloride is the inorganic compound with the formula Fe Cl 3Also called ferric chloride it is a common compound of iron in the 3 oxidation stateThe anhydrous compound is a crystalline solid with a melting point of 3076 C. Uingredient name u ucas number u upercent u uexposure limits u iron oxide red 1309-37-1 92 acgih tlv.
 Source: studylib.net
Source: studylib.net
Health 0 flammability 0 reactivity 0 section ii composition. We know this coat as rust but its full chemical formula is ironIII oxide or Fe²O³. When wet it is corrosive to aluminum and most metals. 32 14 57 52 11 Emergency Number. Iron is an essential component of.
 Source: researchgate.net
Source: researchgate.net
ATS 835 is a great replacement for alum because it does an amazing job but with. There is no set time frame to rusting. Iron and its related alloys react to oxygen and water in a specific way. Not classified Ferric Chloride Hexahydrate 10025 -77 -1. For each subject the hemoglobin incorporation method was used to measure the true absorption of 60 mg of iron from either ferrous sulfate or ferric ammonium citrateThe iron tolerance test ITT was also studied for these two compounds and for reduced iron.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title ferric iron hazards by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.