Fda class iv
Home » chemical » Fda class iv >Fda class iv
Fda Class Iv. With the newer models of high power or high intensity Class IV therapy lasers treatments are now available from a growing number of medical professionals that include physical therapists. I Has an effect on a specific disease or class of diseases. Even staring at the diffuse. The higher the class the more powerful the laser is and the greater the.
 Is Your Software A Medical Device Raps From raps.org
Is Your Software A Medical Device Raps From raps.org
A statement claims to diagnose mitigate treat cure or prevent disease if it claims explicitly or implicitly that the product. PREVENT EYE EXPOSURE Class 4 visible-beam lasers are high-powered. The higher the class the more powerful the laser is and the greater the. Therefore the patients dosing schedule should be adjusted so that an additional dose is administered after each dialysis. A Class 4 laser can cause a significant eye injury if the beam whether direct or reflected enters the eye. This results in a 60 decrease in plasma concentrations following a 6-hour dialysis period.
In determining whether a statement is a disease claim under these criteria FDA will consider the context in which the claim is presented.
For patients who require dialysis the mean plasma half-life of acyclovir during hemodialysis is approximately 5 hours. The FDA recognizes four major hazard classes I to IV of lasers including three subclasses IIa IIIa and IIIb. 2 The variable t in the expressions of emission limits is the magnitude of the sampling interval in units of seconds. The FDA approval of sacubitril plus valsartan was based on results from the PARADIGM-HF clinical trial involving 8442 patients with symptomatic chronic heart failure NYHA Class II-IV and systolic dysfunction1118 This study was stopped early after a median follow-up of 27 months when sacubitril plus valsartan crossed the prespecified margin for a significant reduction in the risks for CV. With the newer models of high power or high intensity Class IV therapy lasers treatments are now available from a growing number of medical professionals that include physical therapists. 1 The factors k 1 and k 2 are wavelength-dependent correction factors determined from table IV.
 Source: danburypodiatrist.com
Source: danburypodiatrist.com
1 The factors k 1 and k 2 are wavelength-dependent correction factors determined from table IV. A statement claims to diagnose mitigate treat cure or prevent disease if it claims explicitly or implicitly that the product. A Class 4 laser can cause a significant eye injury if the beam whether direct or reflected enters the eye. Pharmacokinetic parameters for Cefepime in healthy adult male volunteers n9 following single 30minute infusions IV of Cefepime 500 mg 1 g and 2 g are summarized in Table 7. The FDA recognizes four major hazard classes I to IV of lasers including three subclasses IIa IIIa and IIIb.
 Source: news.iscas.co
Source: news.iscas.co
Even staring at the diffuse. Therefore the patients dosing schedule should be adjusted so that an additional dose is administered after each dialysis. In determining whether a statement is a disease claim under these criteria FDA will consider the context in which the claim is presented. The special control for this device is FDAs Class II Special Controls Guidance Document. The higher the class the more powerful the laser is and the greater the.
 Source: imarcresearch.com
Source: imarcresearch.com
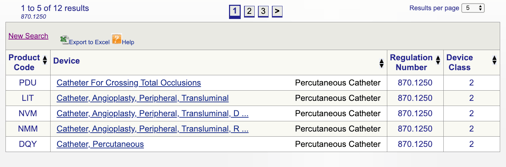
Cefepime pharmacokinetics are. Pharmacokinetic parameters for Cefepime in healthy adult male volunteers n9 following single 30minute infusions IV of Cefepime 500 mg 1 g and 2 g are summarized in Table 7. Percutaneous Transluminal Coronary Angioplasty PTCA Catheters See 8701e for the. 2 The variable t in the expressions of emission limits is the magnitude of the sampling interval in units of seconds. The special control for this device is FDAs Class II Special Controls Guidance Document.
 Source: learn.marsdd.com
Source: learn.marsdd.com
The FDA recognizes four major hazard classes I to IV of lasers including three subclasses IIa IIIa and IIIb. With the newer models of high power or high intensity Class IV therapy lasers treatments are now available from a growing number of medical professionals that include physical therapists. SAFE USE GUIDANCE - GENERAL. 1 The factors k 1 and k 2 are wavelength-dependent correction factors determined from table IV. Class 4 is the same as the Roman numeral Class IV you may see on some lasers labels.
 Source: mindflowdesign.com
Source: mindflowdesign.com
Therefore the patients dosing schedule should be adjusted so that an additional dose is administered after each dialysis. Pharmacokinetic parameters for Cefepime in healthy adult male volunteers n9 following single 30minute infusions IV of Cefepime 500 mg 1 g and 2 g are summarized in Table 7. 1 The factors k 1 and k 2 are wavelength-dependent correction factors determined from table IV. Since the first Class III lasers were cleared by the FDA in 2002 and the first Class IV lasers in 2003 the majority of treatments were performed in a medical office and most often by a chiropractor. 6 Class IV levels of laser radiation are considered to be an acute hazard to the skin and eyes from direct and scattered radiation.

The FDA approval of sacubitril plus valsartan was based on results from the PARADIGM-HF clinical trial involving 8442 patients with symptomatic chronic heart failure NYHA Class II-IV and systolic dysfunction1118 This study was stopped early after a median follow-up of 27 months when sacubitril plus valsartan crossed the prespecified margin for a significant reduction in the risks for CV. 2 The variable t in the expressions of emission limits is the magnitude of the sampling interval in units of seconds. 1 The factors k 1 and k 2 are wavelength-dependent correction factors determined from table IV. A statement claims to diagnose mitigate treat cure or prevent disease if it claims explicitly or implicitly that the product. The higher the class the more powerful the laser is and the greater the.
 Source: emergobyul.com
Source: emergobyul.com
Pharmacokinetic parameters for Cefepime in healthy adult male volunteers n9 following single 30minute infusions IV of Cefepime 500 mg 1 g and 2 g are summarized in Table 7. Even staring at the diffuse. In determining whether a statement is a disease claim under these criteria FDA will consider the context in which the claim is presented. SAFE USE GUIDANCE - GENERAL. Percutaneous Transluminal Coronary Angioplasty PTCA Catheters See 8701e for the.
 Source: raps.org
Source: raps.org
Therefore the patients dosing schedule should be adjusted so that an additional dose is administered after each dialysis. Cefepime pharmacokinetics are. 6 Class IV levels of laser radiation are considered to be an acute hazard to the skin and eyes from direct and scattered radiation. 2 The variable t in the expressions of emission limits is the magnitude of the sampling interval in units of seconds. A statement claims to diagnose mitigate treat cure or prevent disease if it claims explicitly or implicitly that the product.
 Source: imarcresearch.com
Source: imarcresearch.com
A Class 4 laser can cause a significant eye injury if the beam whether direct or reflected enters the eye. Class 4 is the same as the Roman numeral Class IV you may see on some lasers labels. 6 Class IV levels of laser radiation are considered to be an acute hazard to the skin and eyes from direct and scattered radiation. Notes applicable to tables I II-A II III-A. I Has an effect on a specific disease or class of diseases.
 Source: greenlight.guru
Source: greenlight.guru
Since the first Class III lasers were cleared by the FDA in 2002 and the first Class IV lasers in 2003 the majority of treatments were performed in a medical office and most often by a chiropractor. For patients who require dialysis the mean plasma half-life of acyclovir during hemodialysis is approximately 5 hours. Pharmacokinetic parameters for Cefepime in healthy adult male volunteers n9 following single 30minute infusions IV of Cefepime 500 mg 1 g and 2 g are summarized in Table 7. In determining whether a statement is a disease claim under these criteria FDA will consider the context in which the claim is presented. Class 4 is the same as the Roman numeral Class IV you may see on some lasers labels.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title fda class iv by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.