Ethylene glycol solubility in oil
Home » chemical » Ethylene glycol solubility in oil >Ethylene glycol solubility in oil
Ethylene Glycol Solubility In Oil. The terms polyalkylene glycol and polyglycol are used interchangeably. Soluble in cold water hot water acetone. Ethylene glycol is used in the natural gas industry to remove water vapor from natural gas before further processing in much the same manner as triethylene glycol TEG. Ethylene glycol is a synthetic liquid substance that absorbs water.
 Good And Bad Solvents For The Vegetable Oils To Determine The Hansen Download Table From researchgate.net
Good And Bad Solvents For The Vegetable Oils To Determine The Hansen Download Table From researchgate.net
Because of its high boiling point and affinity for water ethylene glycol is a useful desiccantEthylene glycol is widely used to inhibit the formation of natural gas. Why do oil and water not mix At the end of the class you will hopefully see that the reason oil and water do not mix is quite distinct from the reason many other substance do not mix. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when. 4 Polyethylene glycols having a molecular weight of 1000 or above are freely soluble in water. Ethylene glycol is a clear colorless syrupy liquid. Another group of polymers characterized by a high degree of cross-linking resist deformation and solution once.
Fluids - Specific Gravities.
A small amount less than 1 is used to control insects in some stored agricultural products and a very small amount is used in hospitals to sterilize medical. Consult standard literature for details of treatment. PEG-60 hydrogenated castor oils had 349 reported uses with a similar function to PEG-40. If ethanol is used a therapeutically effective blood concentration in the range of 100 - 150 mgdl may be. Simultaneous determination of ethylene glycol propylene glycol 13-butylene glycol and 23-butylene glycol in human serum and urine by wide-bore column gas chromatography. Ethylene Glycol Heat-Transfer Fluid.
 Source: researchgate.net
Source: researchgate.net
And ethylene glycol popular for use as antifreeze pictured in Figure 6 are examples of liquids that are completely miscible with water. See solubility in water acetone. Since it is a liquid it can easily penetrate the soil and contaminate groundwater and nearby streams. Simple at-site detection of diethylene glycolethylene glycol contamination of glycerin and glycerin- based raw. It is the simplest alkene a hydrocarbon with carbon-carbon double bonds.
 Source: machinerylubrication.com
Source: machinerylubrication.com
The product is more soluble in water. Why do oil and water not mix At the end of the class you will hopefully see that the reason oil and water do not mix is quite distinct from the reason many other substance do not mix. A small amount less than 1 is used to control insects in some stored agricultural products and a very small amount is used in hospitals to sterilize medical. Ethylene glycol is a clear colorless syrupy liquid. It is odorless but has a sweet.
 Source: researchgate.net
Source: researchgate.net
The hydrophilic portion of an emulsifier could originate from a variety. And ethylene glycol popular for use as antifreeze pictured in Figure 6 are examples of liquids that are completely miscible with water. Simultaneous determination of ethylene glycol propylene glycol 13-butylene glycol and 23-butylene glycol in human serum and urine by wide-bore column gas chromatography. It is a butyl ether of ethylene glycol and is miscible with water and common organic solvents. It is the simplest alkene a hydrocarbon with carbon-carbon double bonds.
 Source: researchgate.net
Source: researchgate.net
Density is given for the actual state at 25C and for liquid phase at melting point temperature. Simple at-site detection of diethylene glycolethylene glycol contamination of glycerin and glycerin- based raw. The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when. You will also see that the hydrophobic. Well today we are going to ask just this question.

It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester. In cases where several ounces 60 - 100 ml have been ingested consider the use of ethanol and hemodialysis in the treatment. Ethylene Glycol Heat-Transfer Fluid. Another group of polymers characterized by a high degree of cross-linking resist deformation and solution once. Simultaneous determination of ethylene glycol propylene glycol 13-butylene glycol and 23-butylene glycol in human serum and urine by wide-bore column gas chromatography.
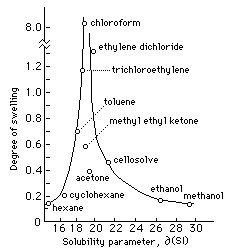 Source: cool.culturalheritage.org
Source: cool.culturalheritage.org
The product is more soluble in water. Ethylene is widely used in the chemical industry and its worldwide production over 150 million tonnes in 2016 exceeds that of any other organic. It is mixed through the etherification and esterification of hydrogenated castor oil glyceride and fatty acid products having sixty equivalents of ethylene oxide. PEG-60 hydrogenated castor oils had 349 reported uses with a similar function to PEG-40. Specific gravities for some common fluids like acetone alcohol turpentine oil and more.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The most common are the water-soluble fluids and thus they can have some very different properties. You will also see that the hydrophobic. The emulsifiers are also described as amphiphilic. Water and antifreeze are miscible. Miscible with lower aliphatic alcohols glycerol acetic acid acetone and simil ar ketones aldehydes pyridine similar.

Mixtures of the two are. It is a butyl ether of ethylene glycol and is miscible with water and common organic solvents. Ethylene glycol is a synthetic liquid substance that absorbs water. This fact is so well engrained in our ever-day experience that we never ask why. Immediate steps should be taken to limit its spread to the environment.
 Source: researchgate.net
Source: researchgate.net
Soluble in cold water hot water acetone. It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester. Two-cycle motor oil is miscible with gasoline. Ethene is a hydrocarbon which has the formula C 2 H 4 or H 2 CCH 2. Slightly soluble in di ethyl ether.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Density is given for the actual state at 25C and for liquid phase at melting point temperature. Water and antifreeze are miscible. Inorganic Compounds in Water - Melting and Boiling Temperatures Densities and Solubility - Physical constants for more than 280 common inorganic compounds. Ethylene oxide is a flammable gas with a somewhat sweet odor. Why do oil and water not mix At the end of the class you will hopefully see that the reason oil and water do not mix is quite distinct from the reason many other substance do not mix.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title ethylene glycol solubility in oil by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.